Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
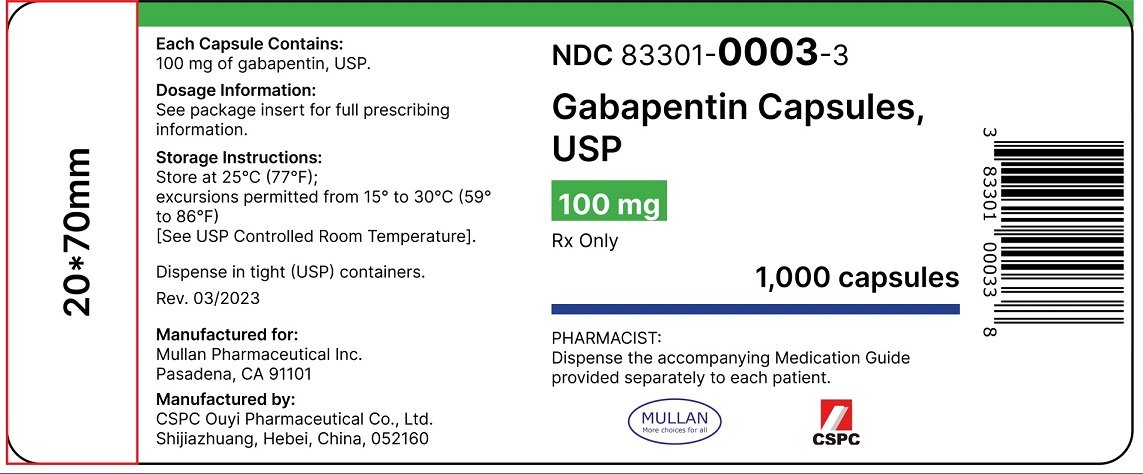
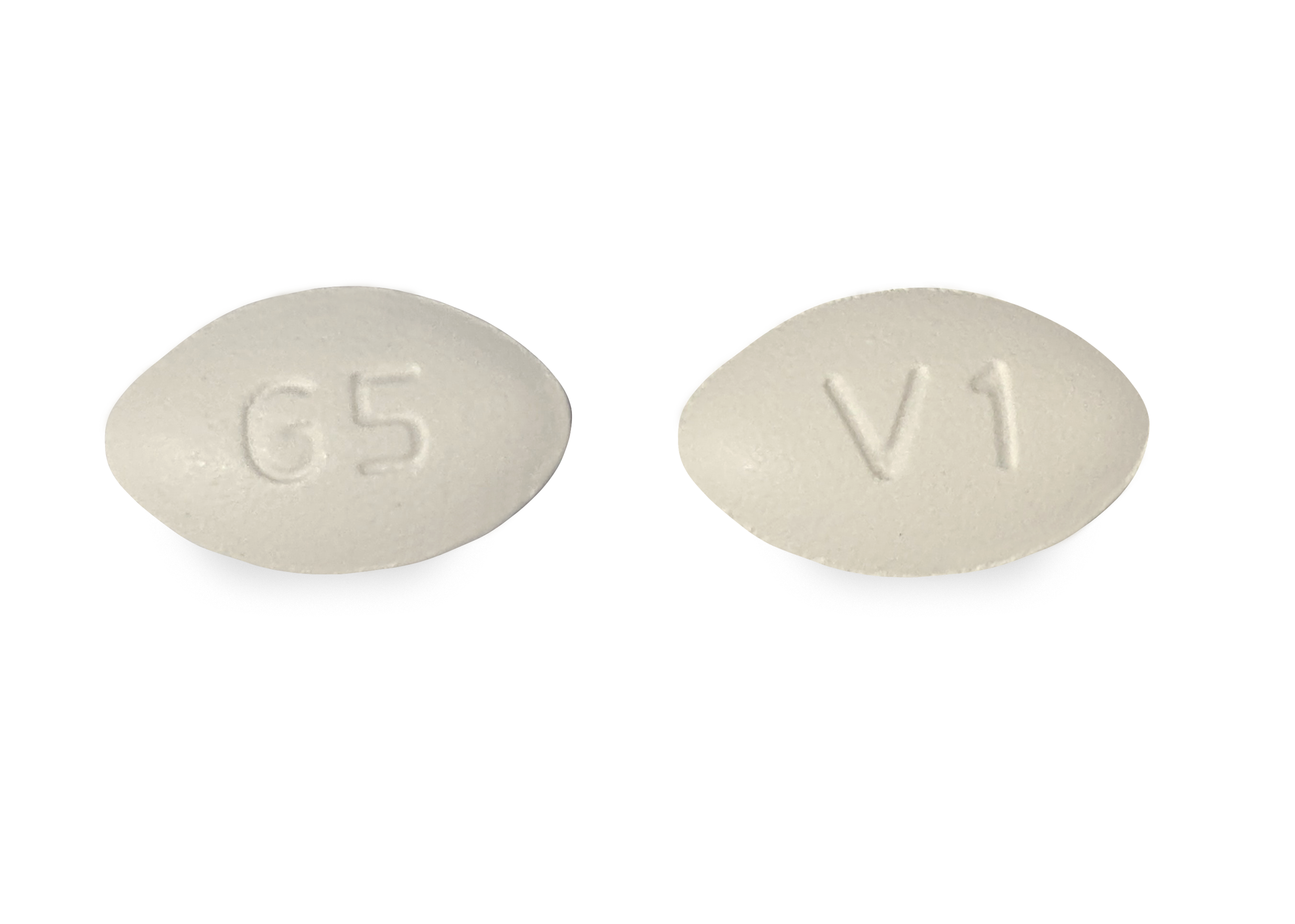
Brand Reference Neurontin Caps; Therapeutic Category Anticonvulsants; Pronunciation GA ba PEN tin; Inactive Ingredients mannitol, pre-gelatinized starch and talc. The 100 mg capsule shell contains titanium dioxide, gelatin and sodium lauryl sulfate. GABAPENTIN- gabapentin solution Amneal Pharmaceuticals LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 INDICATIONS Pfizer, the world's largest drug maker, pleaded guilty on 13 May to numerous civil and criminal charges for illegally promoting the off-label use of gabapentin (Neurontin). It has agreed to pay a $240m (£136m; €200m) criminal fine and $152m to state and federal healthcare programmes. The fine is the second largest given in the industry. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of Gabapentin is recommended for use in focal seizures and neuropathic pain. [7] [10] Gabapentin is prescribed off-label in the US and the UK, [22] [23] for example, for the treatment of non-neuropathic pain, [22] anxiety disorders, sleep problems and bipolar disorder. [24] In recent years, gabapentin has seen increased use, particularly in the Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal All pharmaceutical ingredients are tested using current edition of applicable pharmacopeia. Read and understand label and SDS before handling any chemicals. All Spectrum's chemicals are for manufacturing, processing, repacking or research purposes by experienced personnel only. ACI Healthcare USA, Inc.: Gabapentin Capsules USP are indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of This product information is intended only for residents of the United States. In order to properly inform the public, we have developed the following statement to be placed at the top of certain product pages: Gabapentin is a prescription medicine used to treat: • Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. These highlights do not include all the information needed to use NEURONTIN safely and effectively. See full prescribing information for. NEURONTIN. 35 mg/kg/day, given in three divided doses. Gabapentin by is a Prescription medication manufactured, distributed, or labeled by ACI Healthcare USA, Inc., ACI HealthCare Limited, Strides Pharma Science Limited, Vivimed Life Sciences Private Limited. Drug facts, warnings, and ingredients follow. This label may not be the latest approved by FDA. For current labeling information, please visit particular, gabapentin prevents pain-related responses in 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or Also by this Manufacturer. NDC 70771-1861-9 in bottle of 90 tablets. Gabapentin tablets, 300 mg. Rx only. 90 tablets gabapentin 300 mg NDC 70771-1862-9 in GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 ----- INDICATIONS AND USAGE ----- Gabapentin Oral Solution is indicated for: • Postherpetic neuralgia in adults (1) Store NEURONTIN Capsules and Tablets between 68°F to 77°F (20°C to 25°C). Store NEURONTIN Oral Solution in the refrigerator between 36°F to 46°F (2°C to 8°C). Keep NEURONTIN and all medicines out of the reach of children. General information about the safe and effective use of NEURONTIN
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |