Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
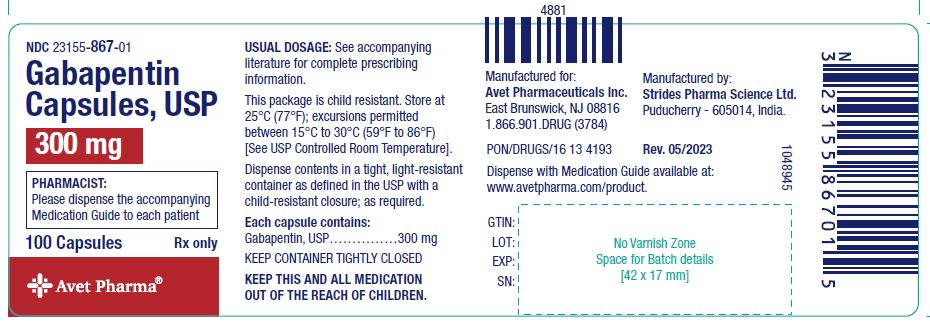
Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Gabapentin is not a federally-controlled drug substance and does not contain an opioid (narcotic) medication. However, gabapentin misuse and abuse has been reported, and it may be restricted in some states through their state drug-monitoring program. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Gabapentin is chemically known as 2-[1-(aminomethyl) cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. Schedule-V controlled substance and mandated reporting to PDMP. The State of Kentucky is, and to date, remains, the only state to have reclassified gabapentin as a Schedule-V controlled substance. 21 Effective July 1, 2017, the prescribing of gabapentin is limited to authorized practitioners, defined as practitioners registered with the US DEA. 21 Thus, mid-level practitioners, specifically On a federal level, gabapentin is not a controlled substance. However, because of increasing numbers of prescriptions and reports of misuse and harm, gabapentin is a controlled substance in some US states, with certain other states requiring gabapentin prescriptions to be reported to state databases. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored prescription drug pursuant to Wis. Stat. § 961.385 (1) (ag). Gabapentin is now listed in Wis. Admin. Code § CSB 4.03 (2) as a monitored prescription drug effective September 1, 2021. Gabapentin Has Not Been Scheduled as a Controlled Substance Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. For individuals applying for their initial controlled substance license, as well as those renewing their controlled substance license, rule changes made to R 338.3135 now require continued training in opioids and controlled substance awareness before applying for the initial license and each subsequent renewal of a controlled substance license. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin is an anticonvulsant drug approved by the Food and Drug Administration (FDA) to treat seizure disorders and neuropathic pain. It isn’t a narcotic or federally controlled substance, but it may be regulated and recognized as a controlled substance in some states for its misuse potential. Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. January 9, 2019 – In an effort to continue to combat the opioid epidemic in Michigan, the Dept. of Licensing and Regulatory Affairs (LARA), with the support of the Michigan Board of Pharmacy, has modified its Pharmacy Rules to categorize Gabapentin as a Schedule 5 controlled substance. Gabapentin – or Neurontin – is a medication commonly As of September 2022, gabapentin was classified as a controlled substance in Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia. 6,7 Adding gabapentin to the list of controlled substances has required providers to have a Drug Enforcement Administrationregistration number to prescribe it, adding another layer of
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |