Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
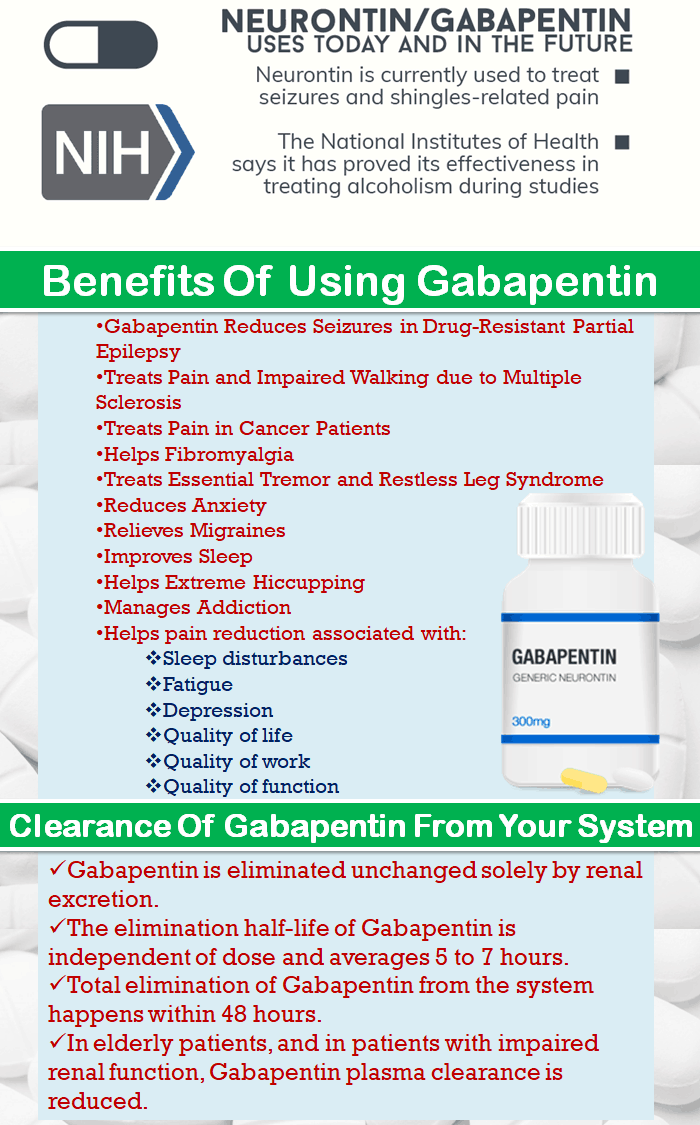
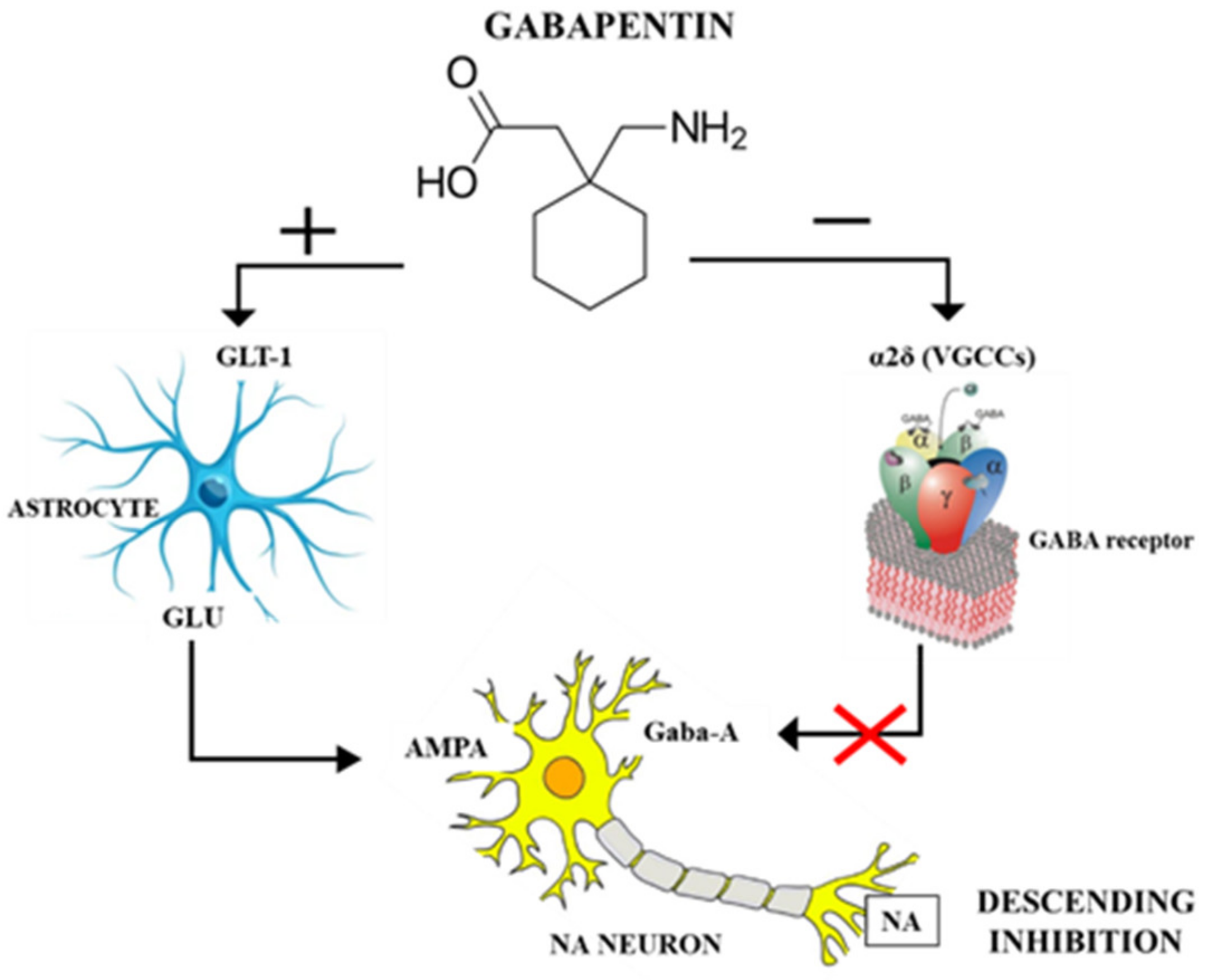
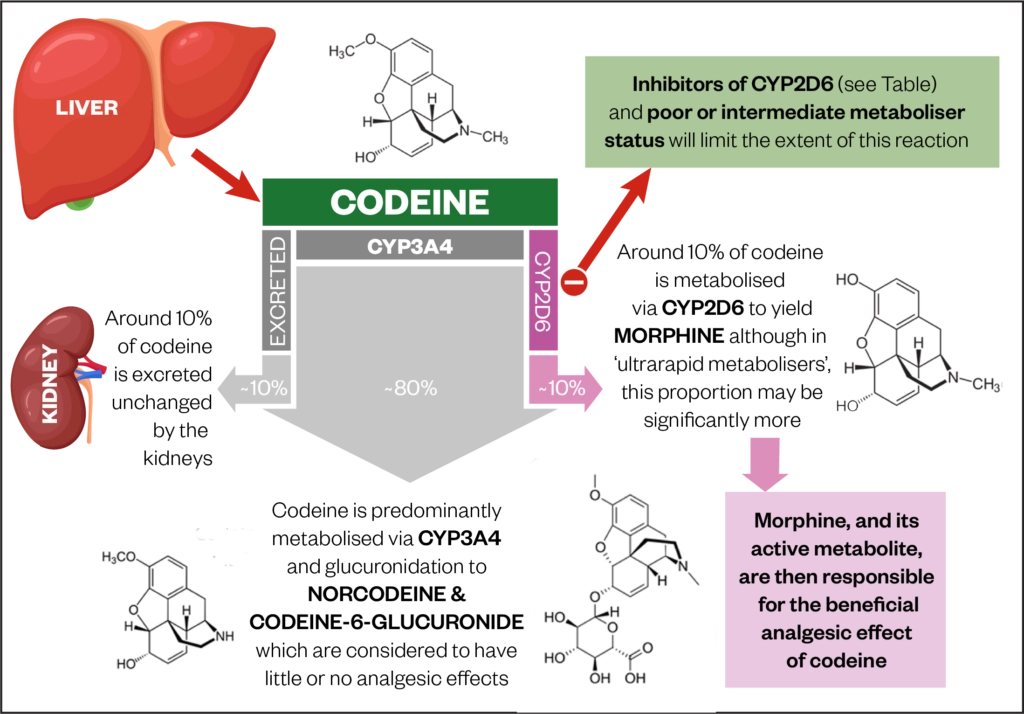
Newer antiepileptic drugs without, or with minimal, hepatic metabolism, such as levetiracetam, lacosamide, topiramate, gabapentin, and pregabalin should be used as first-line therapy. Medications undergoing extensive hepatic metabolism, such as valproic acid, phenytoin, and felbamate should be used as drugs of last resort. Several studies demonstrated that chronic treatment with gabapentin may alter liver and renal homeostasis As no surrogate parameter is available to predict hepatic metabolism of drugs, dose Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gapentin has no appreciable liver metabolism, but suspected cases of gabapentin-induced hepatotoxicity have been reported. Even high doses of gabapentin (400mg/kg) for 30 days do not produce deleterious adverse effects on the liver or haematological parameters. Gabapentin is not protein-bound. A high volume of distribution indicates greater concentration in tissue than in plasma. It is not metabolized and does not induce hepatic enzymes or inhibit metabolism of other antiepileptic drugs. Gabapentin has minimal direct effects on the liver, as it undergoes minimal hepatic metabolism before excretion. However, liver health remains important because any existing liver disease could indirectly alter how gabapentin is processed in the body. Gabapentin is excreted unchanged in humans but is metabolized to N-methyl-gabapentin in dogs. Results in faster elimination and ability for shorter dose intervals in dogs as compared with humans 2 The metabolism of gabapentin has not been studied in cats, but pharmacokinetics demonstrates faster elimination than in humans, with similar Newer antiepileptic drugs without, or with minimal, hepatic metabolism, such as levetiracetam, lacosamide, topiramate, gabapentin, and pregabalin should be used as first-line therapy. Medications undergoing extensive hepatic metabolism, such as valproic acid, phenytoin, and felbamate should be used as drugs of last resort. In most cases, gabapentin doesn’t hurt the liver or kidneys, though proper dosing is important to prevent side effects. Learn how gabapentin affects the liver and kidneys here. Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Gabapentin, a water-soluble amino acid, is eliminated unchanged by the kidneys and there is no appreciable metabolism by the liver. However, there are a few descriptions of gabapentin-related Gabapentin-Induced Liver Toxicity Am J Ther. 2022 Nov-Dec;29(6):e751-e752. doi: 10.1097/MJT.0000000000001208. Epub 2020 Jun 5. Authors Japjot Chahal 1 Therapy with gabapentin is not associated with serum aminotransferase elevations, but several cases of clinically apparent liver injury from gabapentin have been reported. Drug manufacturer Pfizer classifies abnormal liver function tests as an infrequent side effect for gabapentin 5. There is insufficient data to estimate incidence for these or establish whether gabapentin is the sole cause of elevated liver function tests, notes Pfizer. Gabapentin is not metabolized by the liver. They are not metabolised by the liver and do not affect the cytochrome P450 system, major cytochrome P450 system isoenzymes; however, drug-induced hepatotoxicity has been described in case reports. 16 Elimination is mostly done by the kidney and is proportional to the creatinine clearance. Accumulation can cause renal failure resulting in Gabapentin enacarbil and gabapentin are associated with a low rate of transient serum enzyme elevations during treatment and with rare instances of clinically apparent liver injury. Gabapentin enacarbil is a long acting form of gabapentin that is used for restless leg syndrome and for painful postherpetic neuropathy. Hepatic metabolism converts OXC to its active metabolite monohydroxylated derivative. 32 Liver disease has no effect on the pharmacokinetics of OXC and monohydroxylated derivative. 33 OXC has not been associated with hepatotoxicity except for anecdotal case reports, but it can cause a modest elevation of liver enzymes. 34, 35, 36 P53 gene expression, histological, and immunohistochemical examinations were performed in liver and kidney tissue samples. Key findings: Gabapentin increased AST, ALT, LDH, ALP, urea, creatinine, MDA, and p53 gene expression and it reduced GSH. Moreover, gabapentin administration caused structural changes in the hepatic and renal architecture This class, which includes gabapentin and pregabalin, is not metabolized by the liver. Therefore, risks in patients with advanced liver disease are not greatly increased. However, there are case reports of pregabalin‐induced hepatoxicity.4 Gabapentin and pregabalin are renally excreted, so dosages need to be adjusted for renal failure. Gabapentin is an uncommon cause of DILI reported to cause a hepatocellular, cholestatic, or mixed picture of liver injury. Given the limitations of prior cases, we feel our report most closely ties gabapentin use to the resultant transaminase elevation.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |