Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
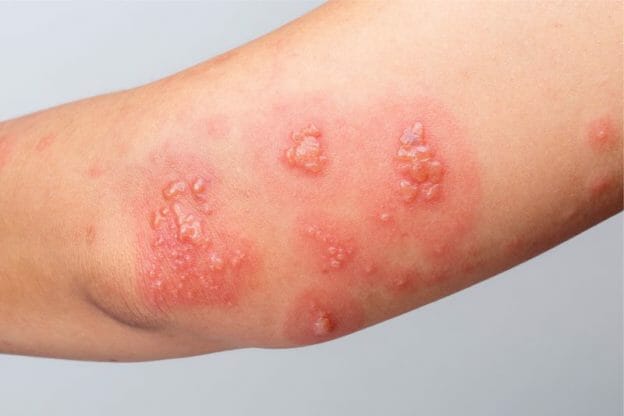 |  |
Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) Microsoft Word - Gabapentin Tablets_BP2017 Author: wallm Created Date: 8/24/2016 10:04:59 AM Guidance Document for Drafting and Formatting of Monographs for Indian Pharmacopoeia; IP Review Process; Stakeholder Comments. New & Revised General Chapter / Monographs - For Comments; Amendments Proposed to IP 2022 - For Comment; Monographs Inclusion-Exclusion Criteria; SOP for Development of IP Monograph ; Meeting of Expert Working Groups Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. United States Pharmacopeia (2024). USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. PRODUCT MONOGRAPH Pr NEURONTIN® Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Pfizer Canada ULC Date of Revision: 17,300 Trans-Canada Highway January 27, 2020 Kirkland, Quebec H9J 2M5 www.pfizer.ca Submission Control No: 232770 ®TM Warner-Lambert Company LLC Pfizer Canada ULC, Licensee ©Pfizer Canada ULC 2019 NEURONTIN® (gabapentin) Product GABAPENTIN (gabapentin) PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrGABAPENTIN Gabapentin Capsules Capsules, 100 mg, 300 mg, and 400 mg, Oral pms-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. A comprehensive guide to gabapentin, an anticonvulsant and analgesic drug. Includes indications, dosage, warnings, interactions, stability, and more. This web page provides the label of Neurontin (gabapentin), a drug used for epilepsy and neuropathic pain. It includes information on gabapentin's mechanism of action, pharmacokinetics, dosage, and adverse reactions. » Gabapentin Tablets contain not less than 90.0 percent age; and L is the Tablet label claim, in mg. and not more than 110.0 percent of the labeled amountTolerances—Not less than 80% (Q) of the labeled amount of of gabapentin (C 9H 17NO 2). gabapentin (C 9H 17NO 2) is dissolved in 45 minutes. TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times 3296Gabapentin / Official Monographs USP 35 Gabapentin RS, USP Gabapentin Related Compound A RS, and.Gabapentin USP Gabapentin Related Compound B RS, respectively. Test solution—Use the Assay preparation. Standard solution—Dissolve a suitable quantity of USP Gabapentin Related Compound E RS in Diluent to obtain a solu- PRODUCT MONOGRAPH PrAPO-GABAPENTIN Gabapentin Capsules Apotex Standard 100 mg, 300 mg and 400 mg Gabapentin Tablets USP 600 mg and 800 mg Antiepileptic Agent APOTEX INC. DATE OF REVISION: 150 Signet Drive April 5, 2018 Toronto, Ontario M9L 1T9 Submission Control No: 214592 Tell your doctor immediately if you experience increased side effects such as drowsiness or slowed breathing while taking GABAPENTIN with an opioid or other sedatives and tranquilizers. The dose of these drugs or GABAPENTIN may need to be adjusted. Avoid alcoholic drinks while taking GABAPENTIN. Gabapentin Page 1 of 28 PRODUCT MONOGRAPH Pr Gabapentin Gabapentin Tablets, House Standard 600 mg and 800 mg Antiepileptic Agent JAMP Pharma Corporation 1310 rue Nobel Boucherville, Quebec J4B 5H3 Control# 256895 GABAPENTIN Product Monograph Page 3 of 32 PrGABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg and 400 mg PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Dosage Form / Strength All Non-medicinal Ingredients Oral Capsules: 100 mg, 300 mg, and 400 mg Corn Starch, Lactose Anhydrous, and Talc. TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Identify the appropriate indications for gabapentin therapy, including neuropathic pain, partial onset seizures, restless legs syndrome, and other relevant neurological and psychiatric conditions. NEURONTIN (gabapentin) Page 1 of 36 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrNEURONTIN® Gabapentin Capsules Capsules, 100 mg, 300 mg and 400 mg, oral Gabapentin Tablets Tablets, 600 mg and 800 mg, oral Antiepileptic Agent BGP Pharma ULC 85 Advance Road Etobicoke (Ontario) Canada M8Z 2S6 Submission Control No.: 274395
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |