Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 | |
 |  |
 |  |
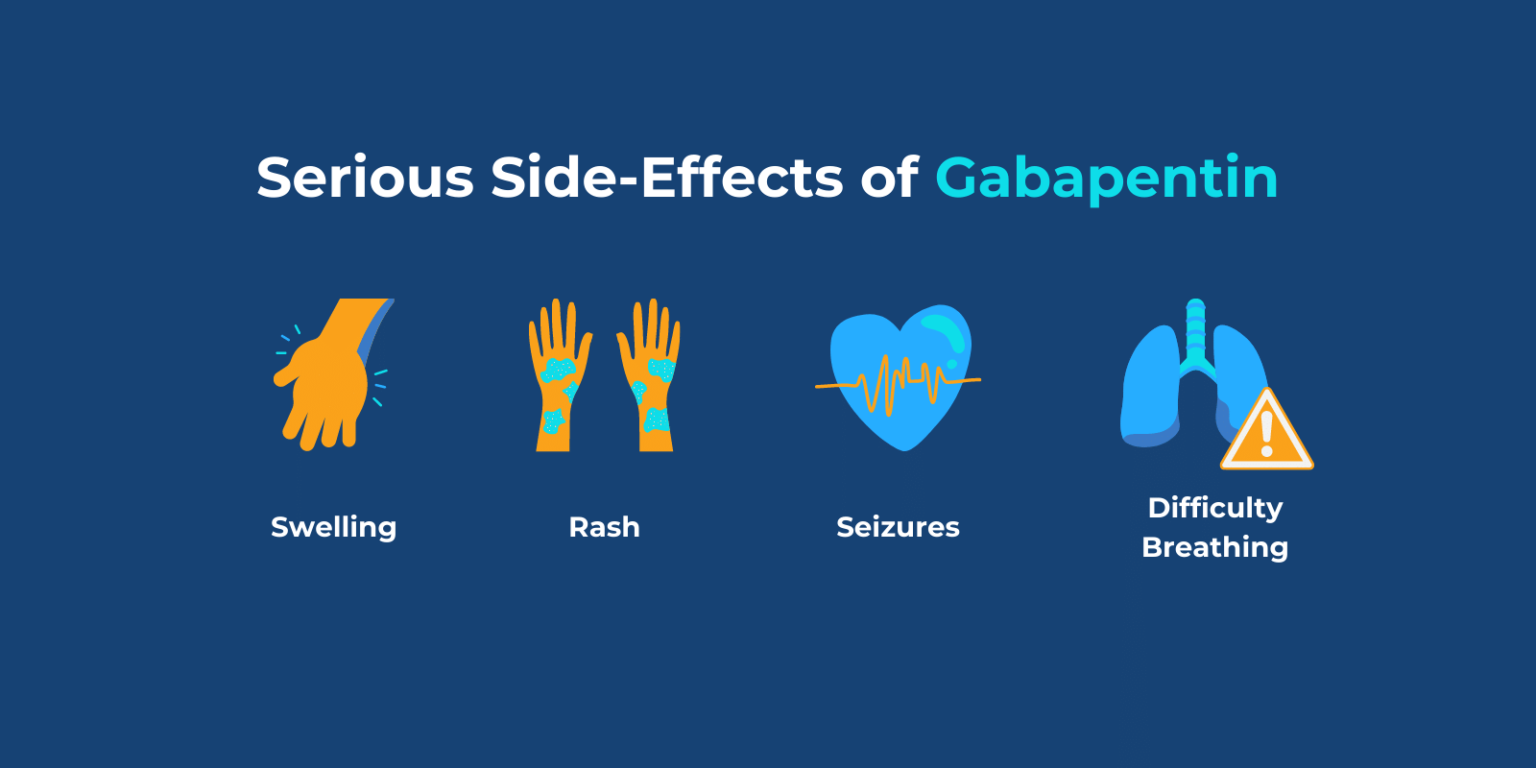
Regardless of the settlement outcome, Gabapentin (brand name “Neurontin”) is still commonly prescribed for a variety of off-label conditions including: fibromyalgia, chronic pain, bipolar disorder, alcohol withdrawal, and migraine headaches. In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 While Gabapentin is FDA-approved for partial seizures and postherpetic neuralgia, its off-label uses are more extensive, especially in psychiatry. Gabapentin for anxiety disorders is notable, with doses between 900 and 3,600 milligrams per day showing effectiveness in reducing symptoms. For gabapentin, the only pain-related indication approved by the US Food and Drug Administration (FDA) is postherpetic neuralgia. For pregabalin, FDA-approved indications related to pain are limited to postherpetic neuralgia, neuropathic pain associated with diabetic neuropathy or spinal cord injury, and fibromyalgia. Brand names include Neurontin and Gralise. Known off-label uses where doctors prescribe gabapentin include as a treatment for hot flashes, restless leg syndrome, Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. With co In many cases, the drugs may be prescribed off label for chronic pain conditions, for which "minimal evidence supporting use" exists, the researchers said. Researchers analyzed data from the Known off-label uses where doctors prescribe gabapentin include as a treatment for hot flashes, restless leg syndrome, mood disorders, anxiety, and to diminish nerve pain related to diabetes or OBJECTIVES: (1) Describe the relevance of off-label use of gabapentin to man-aged care pharmacy; (2) summarize recent FDA warnings and media reports related to off-label gabapentin use; (3) review medical information pertaining to the off-label use of gabapentin; (4) outline alternatives to off-label use of Radley et al. indicated that gabapentin was among the medications with the highest proportion of off-label use, with 83% of its use being off label.9 Gabapentin was initially approved in Canada in April 1994 as adjunctive therapy for the management of epilepsy among patients over 18 years of age who are not controlled by conventional therapy.11 Despite these limited indications, gabapentin and pregabalin are widely prescribed off-label for various other pain syndromes. Such use is growing, possibly because clinicians are searching increasingly for alternatives to opioids. Gabapentinoids, a class of medications including gabapentin (brand name Neurontin) and pregabalin (brand name Lyrica), are among the most prescribed for off-label uses. Gabapentinoids are with approved by the FDA to treat certain specific conditions, such as seizures and fibromyalgia, but 95% of prescriptions are for off-label uses such as low Gabapentin is used to control seizures, to treat nerve pain that can happen after having had shingles, and to treat a condition called restless legs syndrome. In addition to these FDA-approved uses, doctors sometimes prescribe gabapentin off-label. Gabapentin’s off-label uses—meaning prescribing gabapentin for a problem not on the FDA approval list for this medication—that physicians are finding include: Alcohol withdrawal; Anxiety; Cocaine withdrawal; Fibromyalgia; Headaches; Hiccups; Hot flashes; Hyperhidrosis; Insomnia; Migraines; Mood disorders; Pain syndromes; Peripheral Gabapentin was the tenth most commonly prescribed medication in the United States in 2017, and pregabalin ranked sixth in nondiscounted spending for brand-name drugs that same year (with that spending rising from $2.4 to $4.9 billion). For the evidence addressing off-label gabapentinoid use, their noteworthy findings included: An overview of the unapproved use of gabapentin (Substance Abuse: Research and Treatment, Sept. 23, 2018) noted: “Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. Off-label gabapentin (Neurontin) got a bad rep when it missed the mark in bipolar disorder, but there may be something worth salvaging in this drug. Here, we weigh its pros and cons for anxiety, substance use disorders, sleep, pain, and hot flashes, and compare it to its underutilized cousin, pregabalin (Lyrica). There is minimal or no evidence for the use of gabapentin as an off-label therapy for other types of neuropathic pain, low-back pain, radiculopathy, or fibromyalgia. Two doctors recently reviewed published evidence for the benefits and risks of off-label use of gabapentin (originally sold under the trade name Neurontin) and its brand-name cousin Lyrica
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 | |
 |  |
 |  |