Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
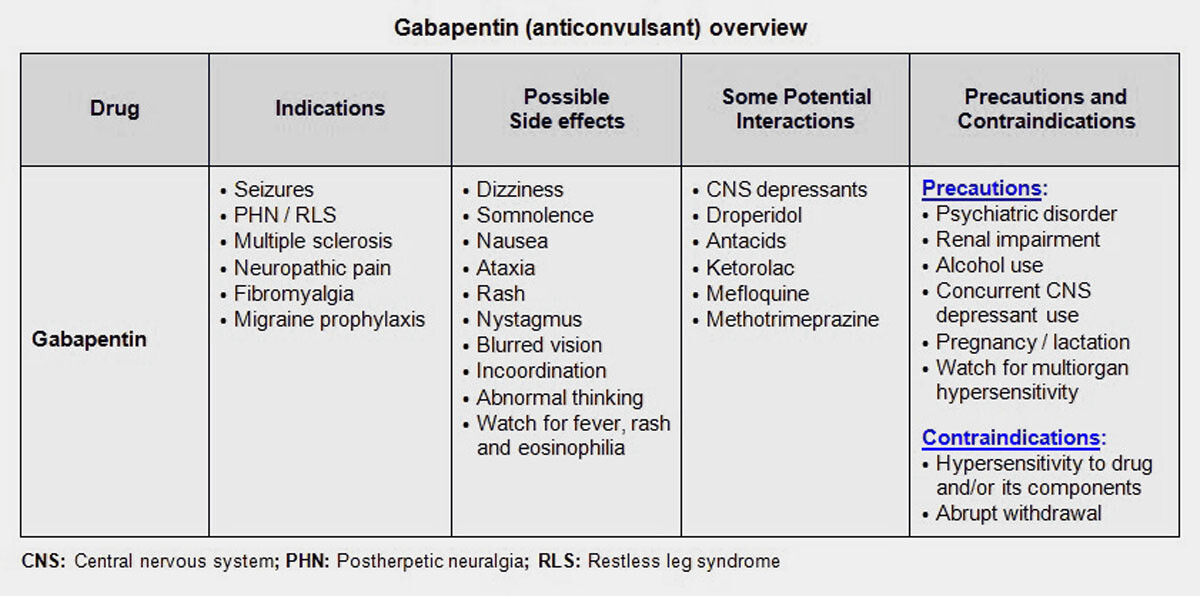
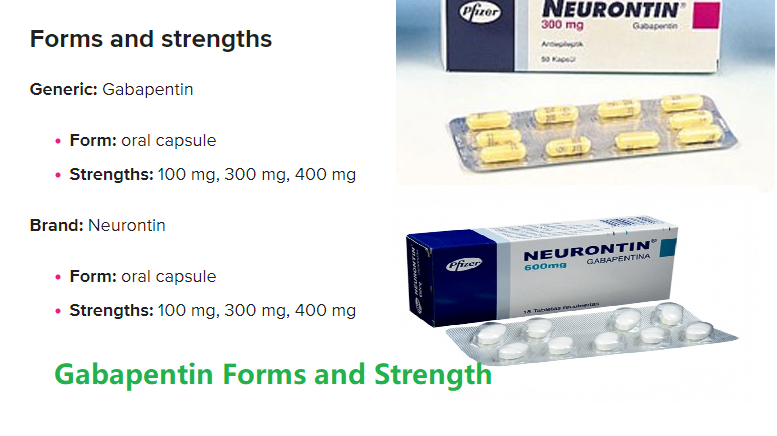
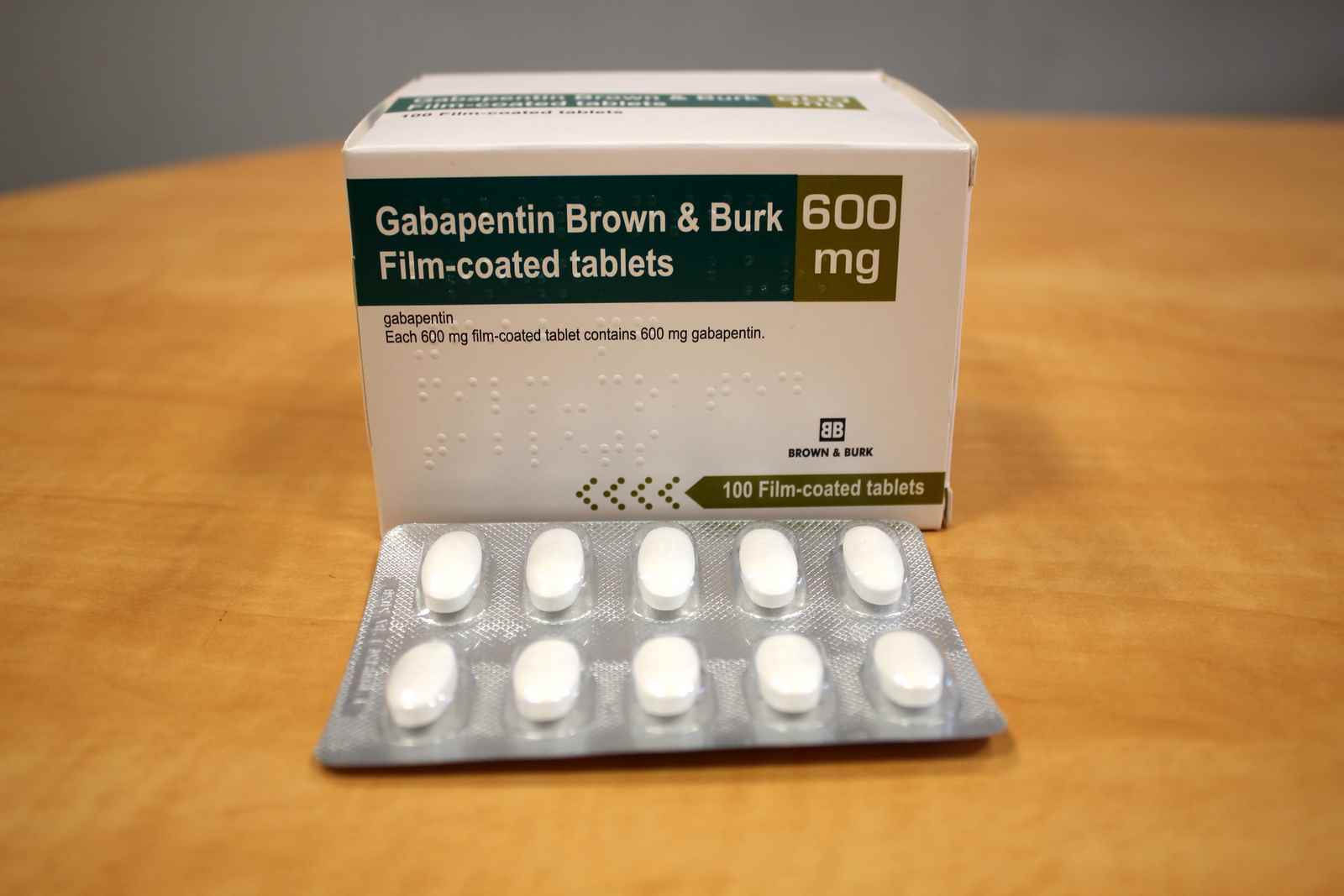
Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia Gabapentin was first approved for use in the United Kingdom in 1993. [16] It has been available as a generic medication in the United States since 2004. [17] It is the first of several other drugs that are similar in structure and mechanism, called gabapentinoids. Two doctors recently reviewed published evidence for the benefits and risks of off-label use of gabapentin (originally sold under the trade name Neurontin) and its brand-name cousin Lyrica Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. When used as directed, gabapentin is known to have numerous uses and benefits. It has been FDA-approved to help control and treat seizures and to diminish a specific type of nerve pain Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. The journey of gabapentin from its original use to its current applications is both fascinating and informative. The Origins of Gabapentin Gabapentin was synthesized in the late 1970s by scientists at Parke-Davis, a pharmaceutical company that is now part of Pfizer. In other words, the patient was first given another anti-convulsant, and then Gabapentin was added. Another study, being done at this time, but as yet incomplete, will establish Neurontin as a mono therapy. Currently, gabapentin is used by some doctors in doses up to 6,000 mg per day. Prescribe first-line medications: Before prescribing gabapentin, psychiatrists should prioritize the use of evidence-based first-line medications for specific symptoms and conditions. Superior agents exist for managing common issues such as anxiety, sleep disturbances, pain, and mood disorders, and should be considered as primary treatment options. Gabapentin, under the brand name Neurontin, was first approved in May 1993 for the treatment of epilepsy in the United Kingdom, and was marketed in the United States in 1994. [43] [44] Subsequently, gabapentin was approved in the United States for the treatment of postherpetic neuralgia in May 2002. [45] Background The gabapentinoid drugs gabapentin and pregabalin were originally developed as antiseizure drugs but now are prescribed mainly for treatment of pain. For gabapentin, the only pain-related indication approved by the US Food and Drug Administration (FDA) is postherpetic neuralgia. Gabapentin’s RLS approval is reserved for gabapentin enacarbil (Horizant), a prodrug that delays absorption by attaching the medication to an enacarbil molecule. However, there are three reasons to prefer the original gabapentin when treating RLS besides the higher cost of the patented Horizant: Convenience. Brown J.N., Wright B.R. Use of gabapentin in patients experiencing hot flashes. Pharmacotherapy, 2009, 29(1), 74-81 Pubmed Burke R.A., Faulkner M.A. Gabapentin enacarbil for the treatment of restless legs syndrome (RLS). Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. In 1993, the FDA approval of Neurontin, the original branded gabapentin, was for use as an adjunctive medication to control partial seizures.9 Over the next several years, the manufacturer, Parke-Davis, a subsidiary of Warner-Lambert, engaged in a large marketing campaign to increase off-label prescribing of Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Carbamazepine was introduced into clinical practice as an antiepileptic drug in 1963. Carbamazepine and lamotrigine block repetitive firing of neurons by blocking sodium channels. Gabapentin, a GABA receptor agonist, was first studied as an antiepileptic drug in humans in 1987 (07). It was launched in the United Kingdom in 1993 and approved in Gabapentin, a gabapentinoid drug, was originally developed as an antiseizure medication. Its primary purpose was to manage and treat epilepsy by stabilizing electrical activity in the brain. Over time, its use has expanded significantly, particularly in the realm of pain management. Gabapentin's Initial Purpose: Antiseizure Medication Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8,10 Gabapentin has
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |