Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
:max_bytes(150000):strip_icc():focal(539x0:541x2)/madonna-tour-071023-02-14734f0027aa4a67a62a73daf8b25b35.jpg) |  |
 |  |
 |  |
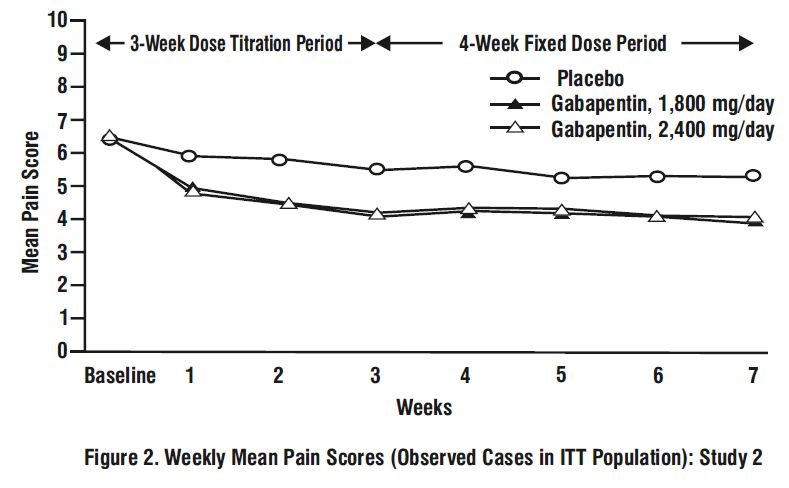
Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. TABLE 1. Gabapentin Tablets Dosage Based on Renal Function; TID = Three times a day; BID = Two times a day; QD = Single daily dose a For patients with creatinine clearance <15 mL/min, reduce daily dose in proportion to creatinine clearance (e.g., patients with a creatinine clearance of 7.5 mL/min should receive one-half the daily dose that patients with a creatinine clearance of 15 mL/min Administer gabapentin orally with or without food. Gabapentin capsules should be swallowed whole with water. If the gabapentin dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). Gabapentin tablets are indicated for: In adults with postherpetic neuralgia, gabapentin tablets may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). REMEDYREPACK INC.: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times (gabapentin extended-release), or Horizant (gabapentin enacarbil extended-release) • If the request is for Lyrica (pregabalin) oral solution, the patient meets ONE of the following: o Has difficulty swallowing oral solid dosage forms (e.g., capsules) An increase in gabapentin AUC values have been reported when administered with morphine. (7.7) An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate release by about approximately 20%, but by only 5% when gabapentin was taken 2 hours after antacids. The effective dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The effective dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. What is NDC 72888-104-25? The NDC Packaged Code 72888-104-25 is assigned to a package of 470 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Advagen Pharma Ltd. The product's dosage form is solution and is administered via oral form. Is NDC 72888-104 included in the NDC Directory? Gralise [package insert]. Newark, CA: Depomed Inc; 2012. Horizant [package insert]. Research Triangle Park, NC: Patheon Inc; 2012. Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280(21):1831-1836. Administer gabapentin orally with or without food. Gabapentin capsules should be swallowed whole with water. If the gabapentin dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). Administer gabapentin tablets orally with or without food. Inform patients that, should they divide the scored 600 mg or 800 mg gabapentin tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividing the scored tablet should be discarded. Gabapentin is indicated for postherpetic neuralgia in adults, adjunctive therapy in the treatment of partial onset seizures with and without secondary generalization in adults and pediatric patients 3 years and older. Patient is allowed the maximum limit for each drug and strength. If the patient is requesting more than the initial quantity limit, the claim will reject with a message indicating that a prior authorization is required. The prior authorization criteria would then be applied to requests submitted for evaluation to the PA unit. Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabarone: Package Insert / Prescribing Info. Package insert / product label Generic name: gabapentin Dosage form: tablet Drug class: Gamma-aminobutyric acid analogs. Medically reviewed by Drugs.com. Last updated on Jan 10, 2025. Gabapentin Tablets package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Administer gabapentin orally with or without food. Gabapentin capsules should be swallowed whole with water. If the gabapentin dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
:max_bytes(150000):strip_icc():focal(539x0:541x2)/madonna-tour-071023-02-14734f0027aa4a67a62a73daf8b25b35.jpg) |  |
 |  |
 |  |