Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
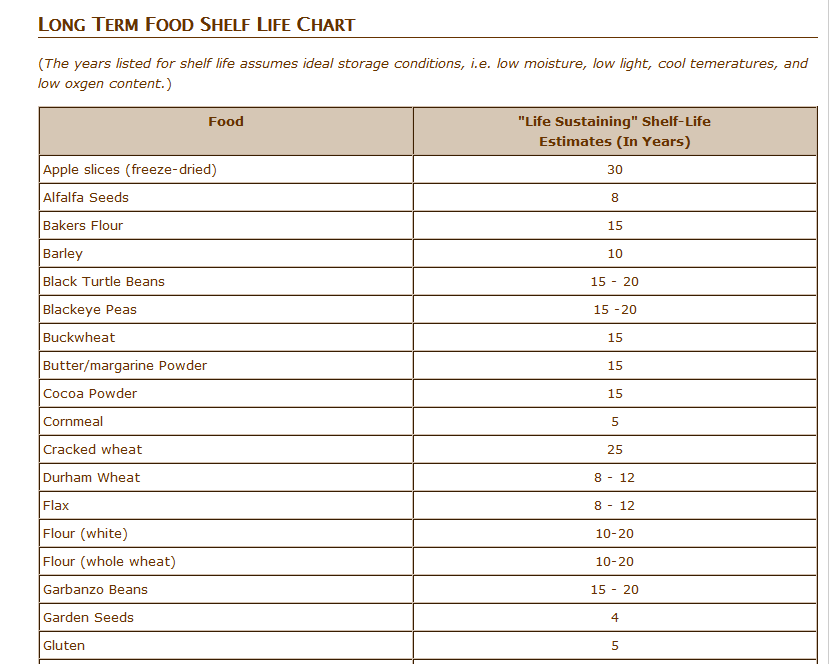
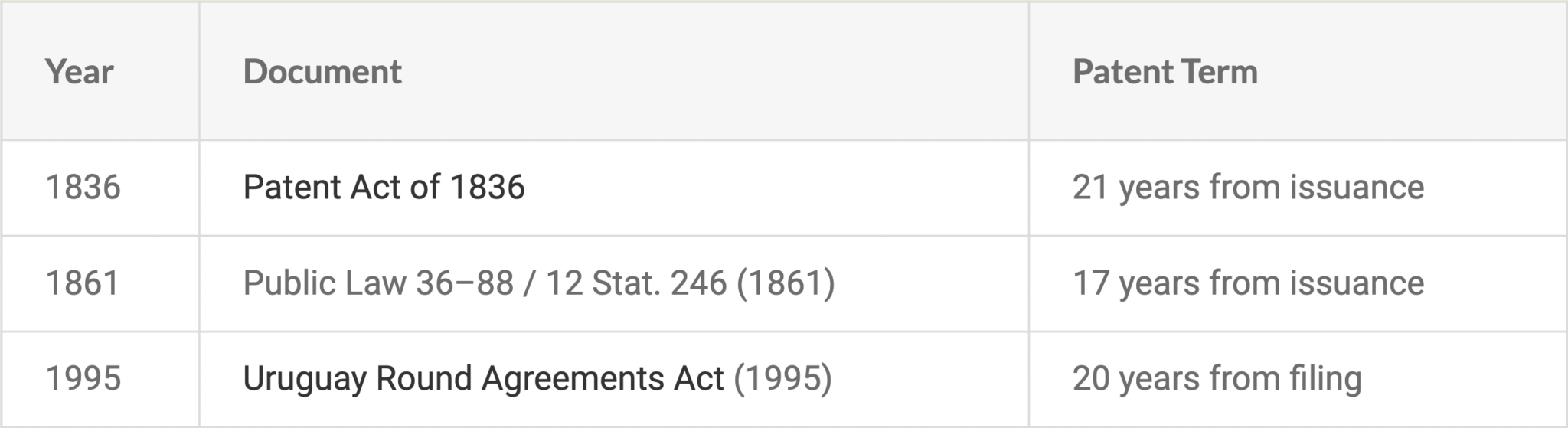
In 2025, a lineup of high-profile drugs will lose their patents, representing billions in sales and years of therapeutic innovation. From life-saving diabetes medications to essential respiratory treatments, these drugs have had a significant impact on modern care. GABAPENTIN ENACARBIL - TABLET, EXTENDED RELEASE;ORAL - HORIZANT. Patent No Patent Expiration Date Patent Title; 6818787: 2025-04-06: Prodrugs of GABA analogs The database provides fast, easy to search and clear reports, to enable the patent expiration of drugs to be effectively and acccurately determined. Users are able to search by date ranges or any other of the included fields indicated below. Approval date: April 18, 2023 Strength(s): 450MG , 750MG , 900MG ; Is there a generic version of Gralise available? Yes. The following products are equivalent to Gralise: gabapentin tablet;oral. Manufacturer: ABON PHARMS LLC Approval date: September 9, 2024 Strength(s): 600MG ; Manufacturer: ANNORA PHARMA Approval date: February 26, 2024 GABAPENTIN INN patent status, expiration and generic drug launch Approval Date Patent No. Patent Expiration; Viatris: NEURONTIN: gabapentin: SOLUTION;ORAL: 021129 Generic name: belinostat DrugPatentWatch ® Estimated Key Patent Expiration / Generic Entry Date: May 13, 2025 Generic Entry Controlled by: Brazil Patent PI0610128 Patent Title: composição farmacêutica, bolsa de infusão intravenosa, frasco ou ampola, kit, usos de um inibidor de hdac e um ou mais de ciclodextrina, arginina e meglumina e de uma composição, e, métodos de regular a The dosage forms are useful for treating or preventing diseases and disorders for which gabapentin is therapeutically effective. Patent expiration dates: June 10, 2029 Information, Expiry & Status of FDA Orange Book Patents covering Gabapentinum A patent's expiry date may change depending upon legal activities going on that patent. Critical activities like abandoning of a patent, term extension of a patent or amendment of its claims can increase or decrease the life of a patent hence affecting its expiry date and in turn affecting the generic launch date of that drug. Is Gabapentin Still Good After 2 Years? A Comprehensive Guide. Understanding Gabapentin Shelf Life and Stability. The Expiration Date Explained; Storage Conditions Matter; Long-Term Use of Gabapentin. Potential Loss of Efficacy; Physiologic Dependence and Withdrawal; Long-term safety concerns; FAQs About Gabapentin Shelf Life and Use; Conclusion Several generic applications have been filed for Gabapentin. The first generic version for Gabapentin was by Actavis Elizabeth Llc and was approved on Sep 12, 2003. And the latest generic version is by Strides Pharma Global Pte Ltd and was approved on Dec 20, 2024. There are twenty-nine drug master file entries for gabapentin. Seventy-five suppliers are listed for this compound. There are two tentative approvals for this compound. Hospitals and pharmacies are required to toss expired drugs, no matter how expensive, vital or scarce. And that's even though the FDA has long known that many remain safe and potent for years longer. A: Payments from gabapentin manufacturers to physicians have been linked to a higher likelihood of prescribing brand-name versions of gabapentin, which are more expensive than generic versions[5]. Q: What challenges does the gabapentin market face? Gabapentin, like all prescription drugs, does expire. Generally, the expiration date of gabapentin after manufacture is usually 2 to 3 years. After dispensing by a pharmacy to the patient, the expiration date is generally set at 1 year (from the date of dispensing). There is no evidence to suggest that gabapentin turns harmful after it has expired. Gabapentin enacarbil is a prodrug of gabapentin and, accordingly, its therapeutic effects in restless legs syndrome and postherpetic neuralgia are attributable to gabapentin. In animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be June 10, 2029. This may change due to patent challenges or generic licensing. Annual sales in 2021 were $171mm indicating the motivation for generic entry (peak sales were $535mm in 2018). Most people don't need the true exp.date because it should be used up in the 6 month period if taken as prescribed.And there are factors once it leaves the pharmacy that can shorten the date like people that do dumb things like keeping their medicine in the bathroom, or a window sill the best place to keep medication that you are holding is in the back of a kitchen cupboard where it is dark Horizant's generic launch date based on the last expiry date of its patents and exclusivities combined is estimated to be Jun 10, 2029 (This date is subject to change depending upon the patent filing activities by the drug owner or exclusivity additions to its drug application) Gabapentin// Gabapentin (brand name Neurontin) is a medication originally developed for the treatment of epilepsy. Presently, gabapentin is widely used to relieve pain, especially neuropathic pain. Gabapentin is well tolerated in most patients, has a relatively mild side-effect profile, and passe
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |