Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
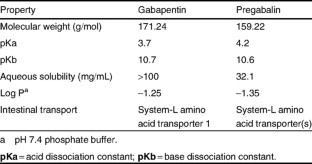
Gabapentinoids are not approved for treatment of fibromyalgia in Australia, but are commonly used. 24 A 2017 systematic review of gabapentin for fibromyalgia pain found only one trial that compared gabapentin with placebo for self-reported pain, and there was no good evidence to support its use. 24 A 2022 meta-analysis of 44 RCTs reported that Pharmacokinetic properties are important to consider in evaluating the usefulness of new antiepileptic drugs (AEDs). Gabapentin is a new, water-soluble, antiepileptic agent with properties of an amino acid. This drug is rapidly absorbed and exhibits dose-dependent bioavailability as a result of a sa This study aimed to describe the pharmacodynamics of gabapentin in the central nervous system of patients with chronic low back pain (CLBP) by using single-photon emission CT (SPECT) with [99mTc]Tc-ECD. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Pharmacodynamics Mechanisms of action. Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs). 19 VGCCs are composed of multiple subunits: α 1, β, γ and α 2 δ. Pregabalin and gabapentin share a similar mechanism of action, inhibiting calcium influx and subsequent release of excitatory neurotransmitters; however, the compounds differ in their pharmacokinetic and pharmacodynamic characteristics. Pregabalin and gabapentin share a similar mechanism of action, inhibiting calcium influx and subsequent release of excitatory neurotransmitters; however, the compounds differ in their pharmacokinetic and pharmacodynamic characteristics. Gabapentin is absorbed slowly after oral administration, with maximum plasma concentrations attained within 3–4 hours. Orally administered gabapentin Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010; 49: 661–669. Gabapentin is regarded as safe and tolerable with a promising pharmacokinetic profile and an extensive therapeutic index. 10 Also, gabapentin have been approved in Japan as adjunctive drug therapy for medically intractable seizures. 4 Pharmacodynamics. Gabapentin is an anti-convulsant medication that inhibits the release of excitatory neurotransmitters, allowing for its use against pathologic neurotransmission such as that seen in neuropathic pain and seizure disorders. 16,19 It has a wide therapeutic index, with doses in excess of 8000 mg/kg failing to cause a fatal Pregabalin and gabapentin share a similar mechanism of action, inhibiting calcium influx and subsequent release of excitatory neurotransmitters; however, the compounds differ in their pharmacokinetic and pharmacodynamic characteristics. This activity outlines the indications, mechanisms of action, administration, significant adverse effects, contraindications, monitoring, and characteristics of gabapentin toxicity. Gabapentin (Neurontin) Primer Gabapentin (Trade name: Neurontin) is an anticonvulsant. Pharmacodynamics. Mechanism of Action. Gabapentin is designed as GABA Gabapentin enacarbil (GEn) is an actively transported prodrug of gabapentin that provides sustained dose-proportional exposure to gabapentin and predictable bioavailability. Gabapentin enacarbil is approved by the US Food and Drug Administration for the treatment of moderate-to-severe primary restle Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st Gabapentin is available in two extended-release for-mulations in addition to the immediate release: a gas-tric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neural-gia. pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin . Pharmacokinet 2010; 49: 661–669. 13. Takahashi Y, Nishimura T, Higuchi K, et al. Transport of pregabalin via L-type . Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. In addition to their use in managing neuropathic pain, gabapentinoids are increasingly being used for off-label conditions despite the lack of evidence. Prescription rates for off-label conditions have overtaken that for on-label use. Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010; 49: 661–669.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |