Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
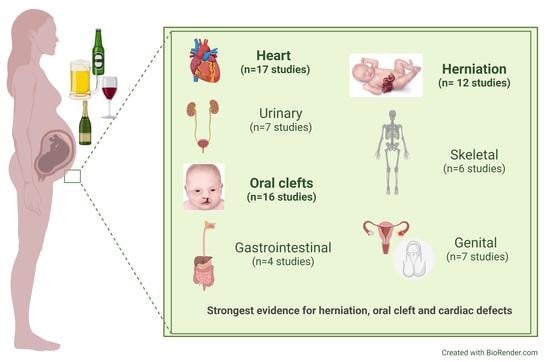
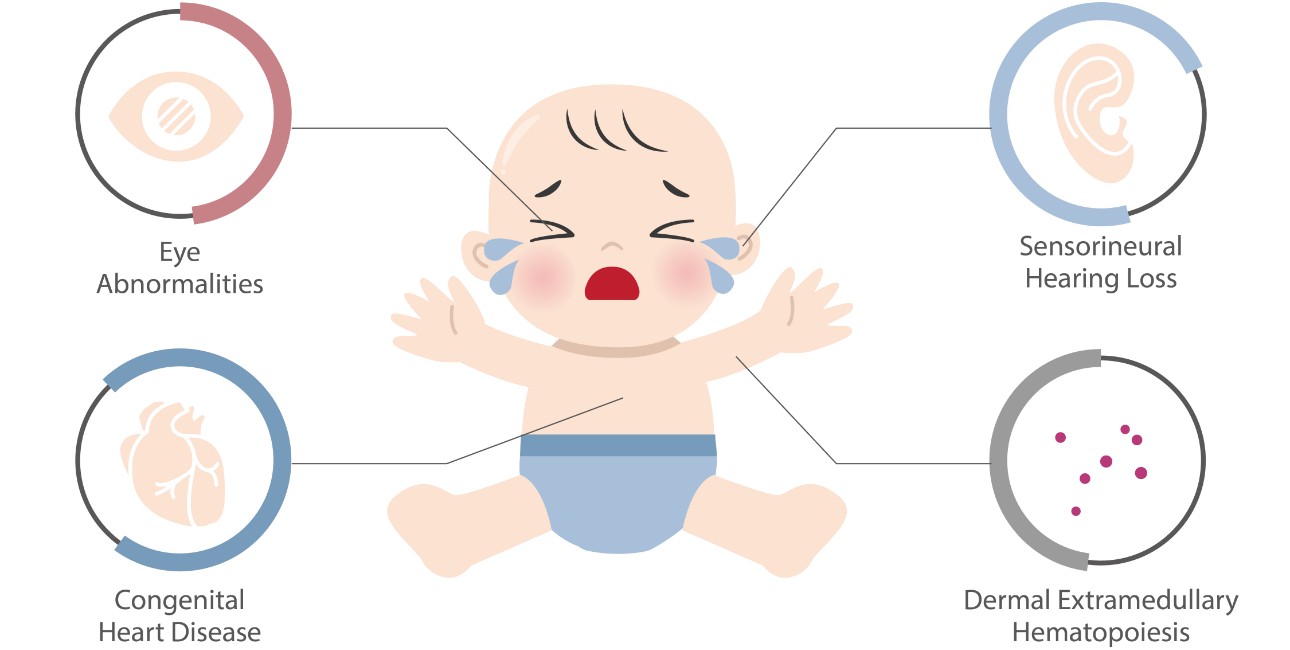
Several studies show that the antiepileptic drug therapy rather than the maternal disease or convulsions is the major cause of malformations identified at birth. Neurontin Birth Defects. All epilepsy drugs carry some risk of birth defects. However, epileptic seizures themselves can also cause damage to a fetus, and an article about seizure medications and pregnancy birth defects featured in the The Los Angeles Times states that “managing a pregnancy in an epileptic woman has generally involved walking a fine line between controlling seizures and There is evidence to suggest that gabapentin use during pregnancy may be associated with an increased risk of certain birth defects. These can range from mild, such as cleft lip or palate, to more severe, such as heart defects or neural tube defects. Does taking gabapentin increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Small, controlled studies on gabapentin have not suggested an increased chance of birth defects. Various studies reported a potential increased risk of malformations with gabapentin use during pregnancy, including cardiac defects. The results of the cohort study and meta-analysis of published evidence support a possible association of congenital malformations and NICU admissions with gabapentin use during pregnancy. Does taking gabapentin increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Small, controlled studies on gabapentin have not suggested an increased chance of birth defects. Some women who took gabapentin during pregnancy say they were never fairly warned about the true risks of birth defects from taking the drug. Some of these women have chosen to file gabapentin birth defect lawsuits against the drug maker for negligence and failure to warn, seeking compensation for medical bills, pain and suffering, and more. Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Small, controlled studies on gabapentin have not suggested an increased chance of birth defects. There is also no known pattern of birth defects associated with the use of gabapentin in pregnancy. The researchers reported 2 major malformations in infants exposed to gabapentin in the first trimester of pregnancy. 3 In another group of 7 women with hyperemesis gravidarum, 2 congenital defects were reported. 4 A cohort study in Denmark reported on 59 fetuses exposed to gabapentin during pregnancy, and documented 1 major malformation and 6 All pregnant women in the UK will be offered a very detailed anomaly scan at around 20 weeks of pregnancy as part of their routine antenatal care. No extra monitoring for major birth defects is required following gabapentin use in pregnancy. Babies exposed to gabapentin before delivery may experience withdrawal symptoms for a few days after birth. We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. A moderate amount of clinical data exist on the risk of birth defects following the use of gabapentin during pregnancy with the available studies only involving around 600 pregnancies exposed to Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Small, controlled studies on gabapentin have not suggested an increased chance of birth defects. There is also no known pattern of birth defects associated with the use of gabapentin in pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as gabapentin, during pregnancy. Encourage women who are taking gabapentin during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling the toll-free number 1-888-233-2334 or Five studies reported significant findings with increased risks of overall congenital anomalies, specific anomalies (nervous system, eyes, oro-facial clefs, urinary and genital system), miscarriage, stillbirth and specific neurodevelopmental outcomes after exposure to pregabalin during pregnancy. There was a higher risk of preterm birth among women exposed to gabapentin either late (RR, 1.28 [1.08-1.52], p < 0.01) or both early and late in pregnancy (RR, 1.22 [1.09-1.36], p < 0.001), SGA We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early Context Epilepsy during pregnancy is a therapeutic challenge. Since the 1990s, the number of licensed antiepileptic drugs has substantially increased, but safety data on first-trimester use of newer-generation antiepileptic drugs and birth defects are limited.Objective To study the association Compared with the general population, women with epilepsy who take antiepileptic drugs during pregnancy may have a higher risk of having a baby who is born with a birth defect or the antiepileptic drug may affect how the baby grows in the womb and the child’s brain development (thinking, language, attention, social and behavioural skills). No elevated risks for birth defects were associated with lamotrigine, levetiracetam, carbamazepine, oxcarbazepine and gabapentin. The researchers caught weak signals linking clonazepam to a higher
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |