Gallery
Photos from events, contest for the best costume, videos from master classes.
 | .jpg) |
 | |
 |  |
 |  |
 |  |
 |  |
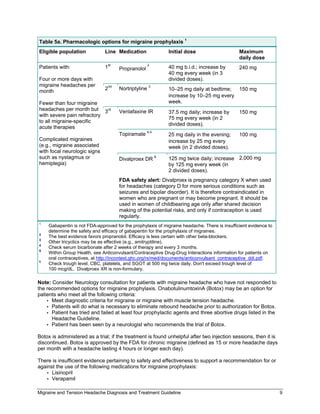
Gabapentin may cause side effects such as dizziness, drowsiness, and dizziness. It is important to follow the prescribed dosage and seek medical attention if experiencing serious side effects or changes in mood or behavior. Gabapentin is prescribed by healthcare professionals and should only be taken under medical supervision. The objective of this study was to assess the safety of gabapentin (Neurontin) exposure in human pregnancy. Prospective and retrospective data concerning 51 fetuses, including 3 twin gestations, were collected from 39 women with epilepsy and other disorders exposed to gabapentin during pregnancy. Does taking gabapentin in pregnancy increase the chance of other pregnancy-related problems? Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. 4,642 pregnancies with first trimester gabapentin exposure were identified (mean age of 28 years; 69% white). A reference group consisting of 1,744,447 unexposed pregnancies (mean age of 24 years; 40% white) was also identified. We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. The majority of the available epidemiological data relate to gabapentin use in pregnancy for the treatment of maternal epilepsy. A few case reports/series describe use of gabapentin in the treatment of neuropathic pain or hyperemesis gravidarum but studies have not assessed fetal outcomes following use in pregnancy for these indications. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early Animal studies have shown that gabapentin intake during pregnancy increases the risk of embryo fetal toxicity, specifically on mice. For rats, adverse effects on offspring development were common among those who had gabapentin during pregnancy. In this large population-based study, we did not find evidence for an association between gabapentin exposure during early pregnancy and major malformations overall, although there was some evidence of a higher risk of cardiac malformations. Maternal use of gabapentin, particularly late in pregnancy Gabapentin is a γ-aminobutyric acid analog formally indicated for the treatment of epilepsy and neuropathic pain that is gaining increased popularity. Gabapentin has been historically considered a safe medication, including during pregnancy and lactation, with low reported concerns for misuse and use disorders. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 The U.S. Food and Drug Administration classifies gabapentin (Neurontin) as a Pregnancy Category C medication, which means that animal studies conducted on this medication has caused harm on the fetus. Selected References: Blotiere PO, et al. 2020. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open 10(6). Brannon GE, Rolland PD. Anorgasmia in a patient with bipolar disorder type 1 treated with gabapentin. J Clin Psychopharmacol. 2000;20(3):379 This article summarizes the current literature regarding gabapentin use during pregnancy and related prenatal and neonatal exposure outcomes with special consideration for interactions between gabapentin and opioid use. Medications approved prior to June 29, 2001 are not subject to the PLLR rule; however, the pregnancy letter category must be removed by June 29, 2018. For generic drugs, if the labeling of a reference listed drug is updated as a result of the final rule, the abbreviated new drug application (ANDA) labeling must also be revised. All pregnant women in the UK will be offered a very detailed anomaly scan at around 20 weeks of pregnancy as part of their routine antenatal care. No extra monitoring for major birth defects is required following gabapentin use in pregnancy. Babies exposed to gabapentin before delivery may experience withdrawal symptoms for a few days after birth. Despite the widespread use, only sparse information is available on the safety of gabapentin during pregnancy. We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes. This sheet is about exposure to gabapentin in pregnancy and while breastfeeding. This information is based on available published literature. It should not take the place of medical care and advice from your healthcare provider. Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. Advice and warnings for the use of Gabapentin during pregnancy. FDA Pregnancy Category C - Risk cannot be ruled out
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | .jpg) |
 | |
 |  |
 |  |
 |  |
 |  |