Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
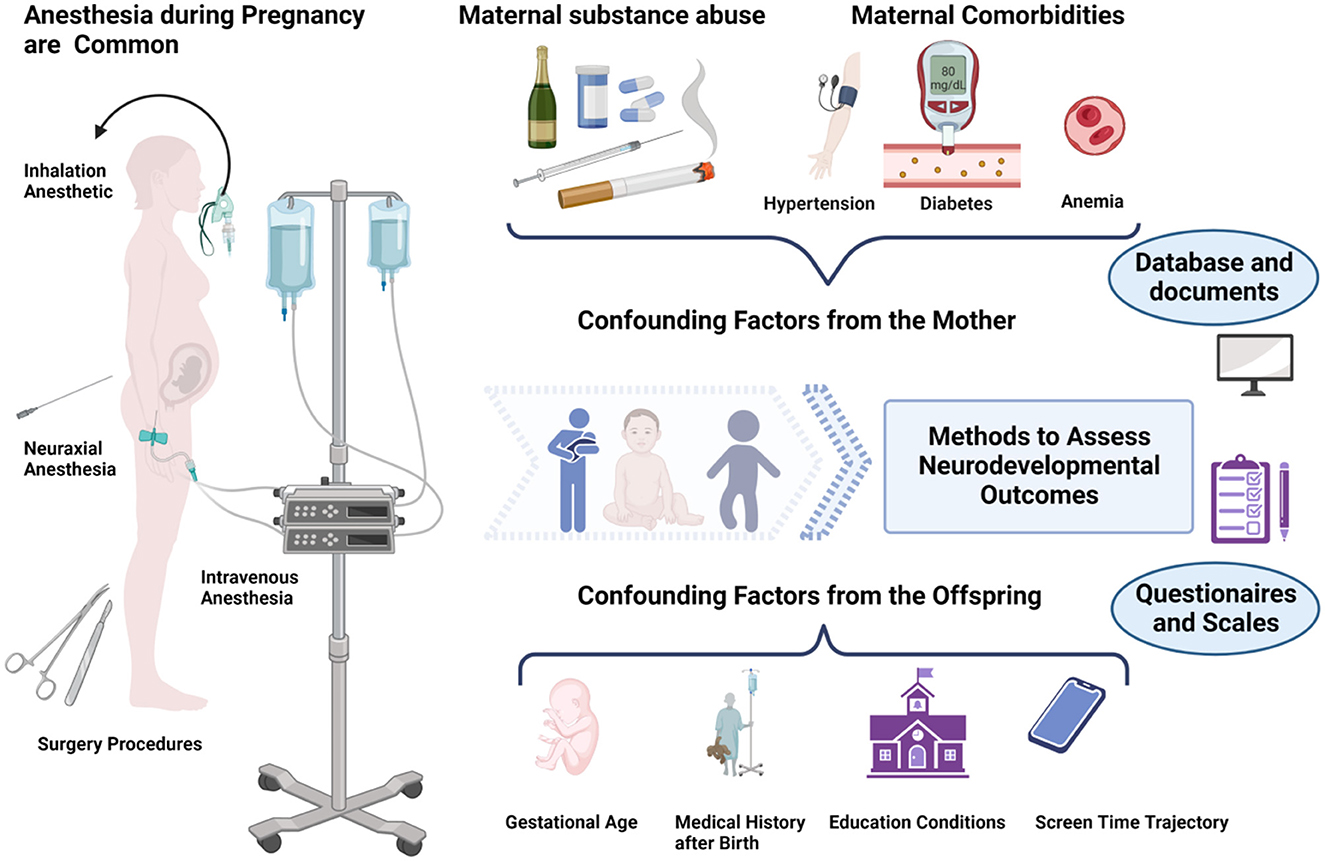
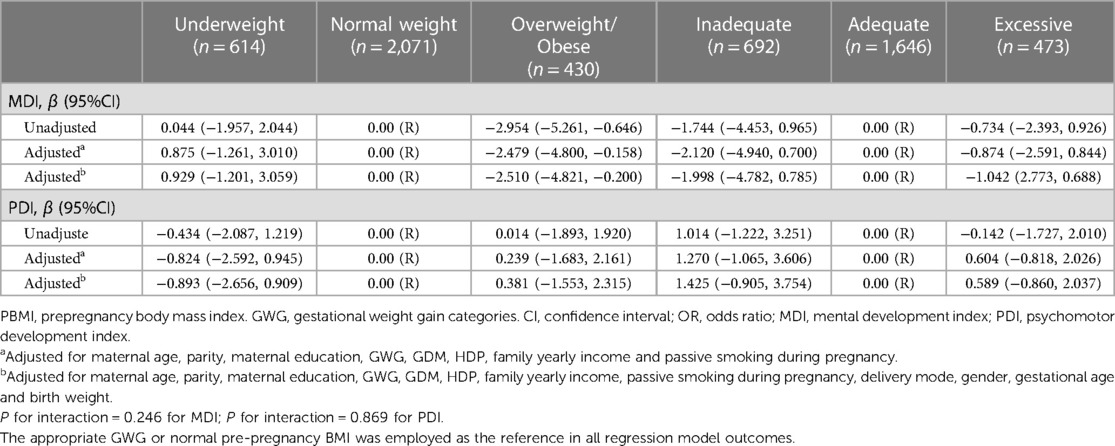
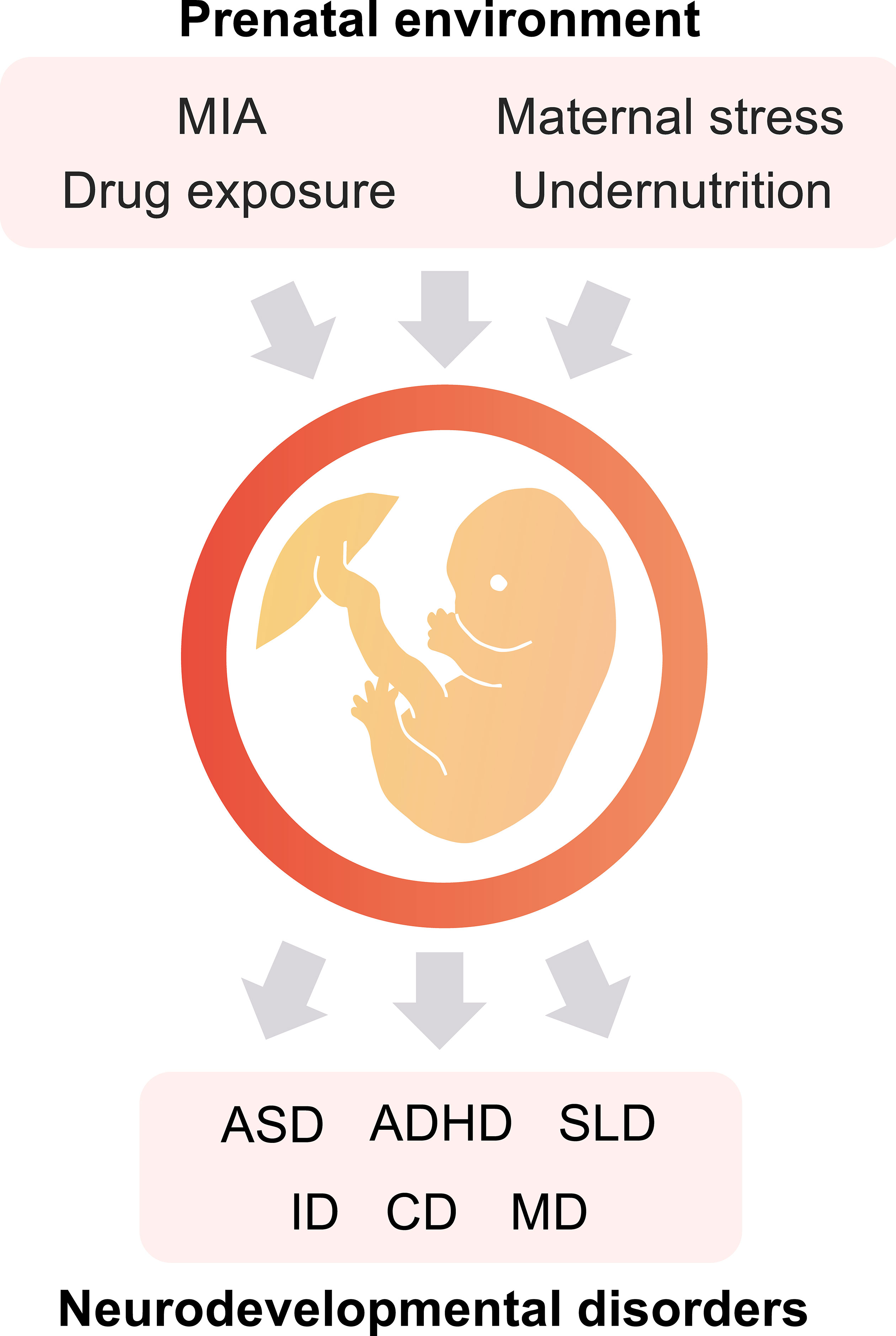
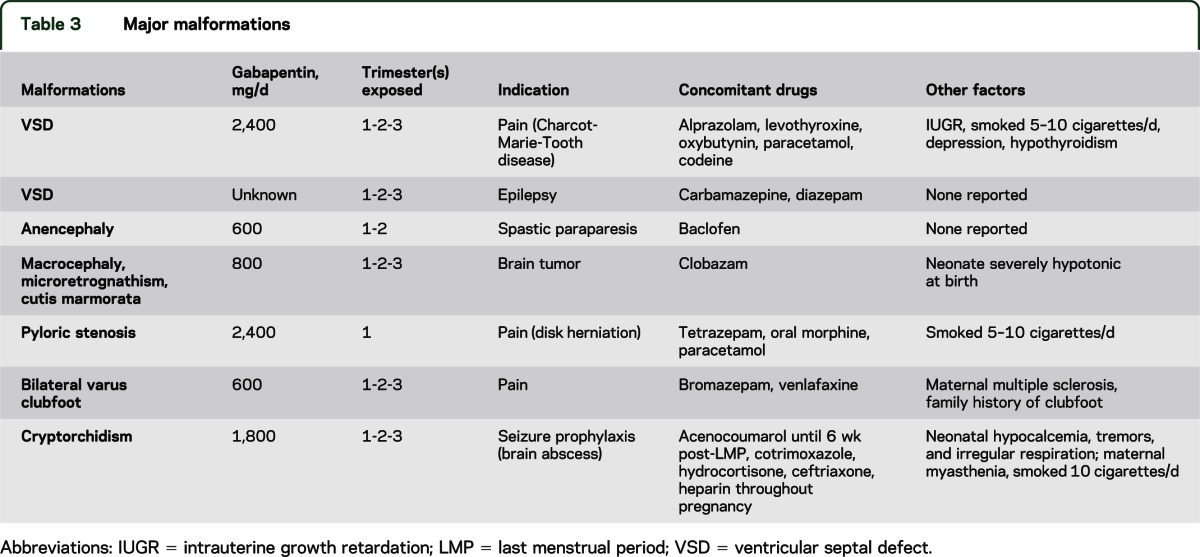
We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. This sheet is about exposure to gabapentin in pregnancy and while breastfeeding. This information is based on available published literature. It should not take the place of medical care and advice from your healthcare provider. What is gabapentin? Gabapentin is a medication that has been used to prevent and control partial seizures, treat some forms [] All pregnant women in the UK will be offered a very detailed anomaly scan at around 20 weeks of pregnancy as part of their routine antenatal care. No extra monitoring for major birth defects is required following gabapentin use in pregnancy. Babies exposed to gabapentin before delivery may experience withdrawal symptoms for a few days after birth. The study supports the reproductive safety of gabapentin during pregnancy. Five studies reported significant findings with increased risks of overall congenital anomalies, specific anomalies (nervous system, eyes, oro-facial clefs, urinary and genital system), miscarriage, stillbirth and specific neurodevelopmental outcomes after exposure to pregabalin during pregnancy. In 2 studies identifying prenatal topiramate exposure from health register data, one found a more than 5-fold increased risk of learning disability, 36 whereas the other identified no abnormal neurodevelopment. 9 However, in the latter study, 75% of the included mothers only filled topiramate prescriptions before or very early in the pregnancy The objective of this study was to assess the safety of gabapentin (Neurontin) exposure in human pregnancy. Prospective and retrospective data concerning 51 fetuses, including 3 twin gestations, were collected from 39 women with epilepsy and other disorders exposed to gabapentin during pregnancy. A study from the European Gabapentin Registry included prospective and retrospective data with a total of 51 outcomes and 44 live births of women with epilepsy and other disorders exposed to gabapentin during pregnancy. Five studies reported significant findings with increased risks of overall congenital anomalies, specific anomalies (nervous system, eyes, oro-facial clefs, urinary and genital system), miscarriage, stillbirth and specific neurodevelopmental outcomes after exposure to pregabalin during pregnancy. We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes. Using the United States Medicaid Analytic eXtract (MAX) dataset, we conducted a population-based study of 1,753,865 Medicaid-eligible pregnancies between January 2000 and December 2013. The majority of the available epidemiological data relate to gabapentin use in pregnancy for the treatment of maternal epilepsy. A few case reports/series describe use of gabapentin in the treatment of neuropathic pain or hyperemesis gravidarum but studies have not assessed fetal outcomes following use in pregnancy for these indications. One study that looked at 378 children exposed to gabapentin during pregnancy did not find an increased chance of conditions that affect how the brain works (neurodevelopmental disorders), conditions that cause problems with social 1 INTRODUCTION. Certain older antiseizure medications (ASMs) act as teratogens, 1, 2 causing harm to the developing brain and leading to poorer neurodevelopmental outcomes in childhood. 3 Mounting evidence regarding older ASM exposure (e.g., valproate, phenobarbital) has prompted changes to prescribing guidelines. 4 This has, in turn, facilitated better evidence-based prescribing and There was an increased risk of preterm birth among women exposed to gabapentin either late (RR=1.28 [CI 1.08-1.52], p < 0.01) or both early and late in pregnancy (RR=1.22 [1.09-1.36], p < 0.001). We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes. Using the United States Medicaid Analytic eXtract (MAX) dataset, we conducted a popula-tion-based study of 1,753,865 Medicaid-eligible pregnancies between January 2000 and December 2013. Gabapentin Pregnancy and Breastfeeding Warnings. Brand names: Fanatrex, Gabarone, Gralise, Neurontin. Pregnancy Warnings; Breastfeeding Warnings; Gabapentin Pregnancy Warnings. Benefits should clearly outweigh risks AU TGA pregnancy category: B3 US FDA pregnancy category: Not assigned One study that looked at 378 children exposed to gabapentin during pregnancy did not find an increased chance of conditions that affect how the brain works (neurodevelopmental disorders), conditions that cause problems with social and communication skills (pervasive developmental disorders), intellectual disability, or communication-related All pregnant women in the UK will be offered a very detailed anomaly scan at around 20 weeks of pregnancy as part of their routine antenatal care. No extra monitoring for major birth defects is required following gabapentin use in pregnancy. Babies exposed to gabapentin before delivery may experience withdrawal symptoms for a few days after birth. Our findings suggest that gabapentin usage in pregnant women, even at therapeutic levels, interferes with the neurogenesis and morphogenesis of vmDA neurons during fetal brain development. Furthermore, several genes involved in the influence of gabapentin on vmDA development were identified.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |