Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
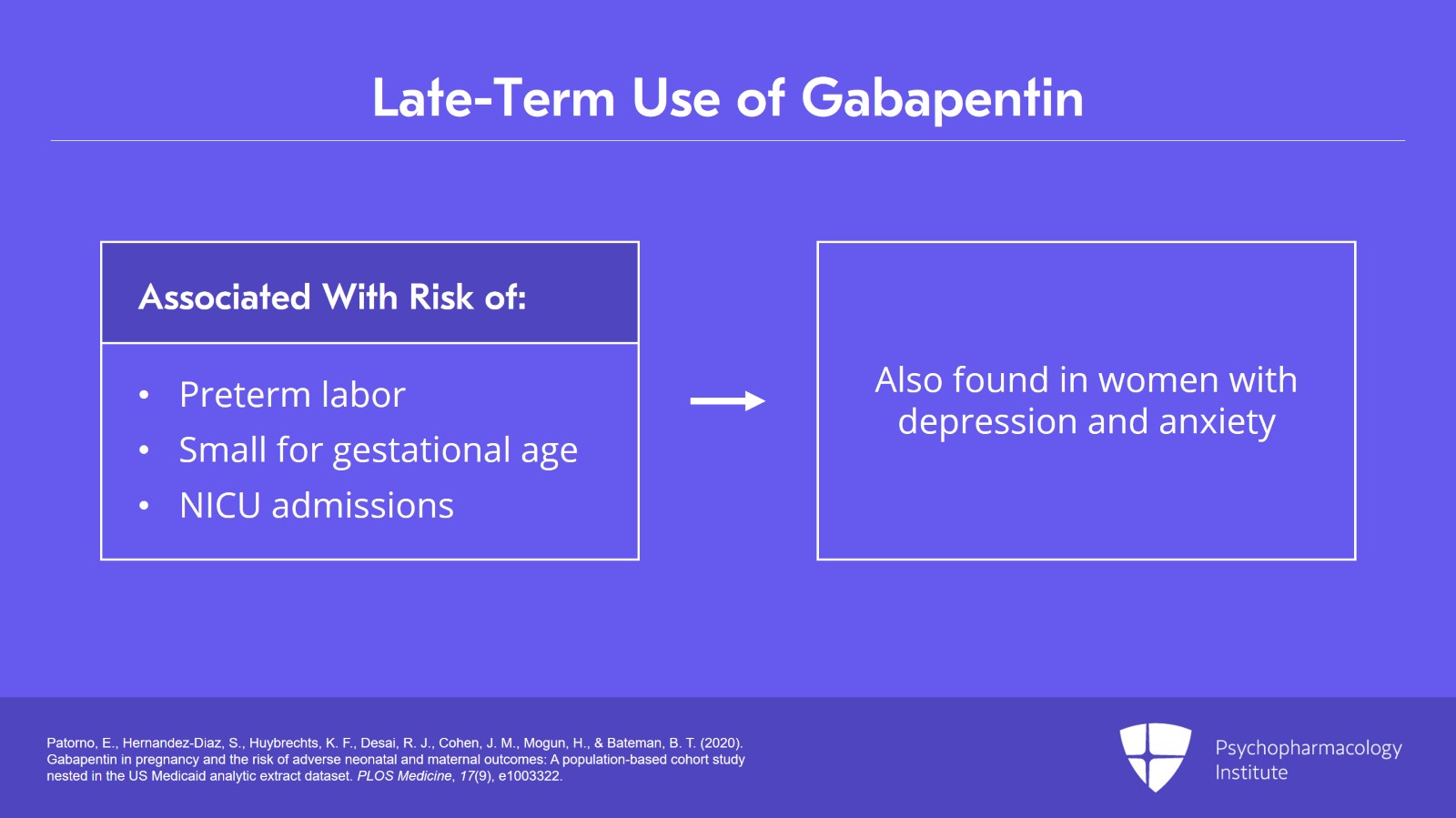
When treating neuropathic pain in a woman who is pregnant, the use of gabapentinoids (e.g. gabapentin) or an antiepileptic drug (AED) (e.g. levetiracetam, lamotrigine) is a last line option. This is due to the limited availability of data for safe use during pregnancy. Other options should be trialled first. In this large population-based study, we did not find evidence for an association between gabapentin exposure during early pregnancy and major malformations overall, although there was some evidence of a higher risk of cardiac malformations. Maternal use of gabapentin, particularly late in pregnancy While the available information does not strongly suggest that it causes problems for the baby, further research is required to prove that gabapentin is safe. As a precaution, gabapentin is only prescribed in pregnancy when the benefits (most commonly of controlling seizures in women with epilepsy) outweigh the possible risks. In a cohort study of pregnant women included in the US Medicaid Analytic eXtract (MAX) dataset, Elisabetta Patorno and colleagues investigate neonatal and maternal outcomes associated with gabapentin exposure during pregnancy. While gabapentin (Neurontin) is now used in a wide variety of clinical settings — for epilepsy, pain management, restless leg syndrome, anxiety, and sleep disturbance – there is relatively little information regarding its reproductive safety. We found that gabapentin could induce high expression of dopaminergic related genes, which suggested that gabapentin use might not be safe during pregnancy. Gabapentin is commonly used as a first-line treatment and is the most frequently prescribed drug in neuropathic disorders. The misuse of gabapentin is known to cause serious negative effects. Gabapentin is not generally recommended in pregnancy as there is not enough information about whether it's safe for your baby. However, from the small amount of information that is available, there's no clear evidence that it's harmful. Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. Data on gabapentin use in pregnancy are mixed—some studies suggest it can result in increases in birth defects and other pregnancy complications, while other studies suggest it’s safe. Since decisions about gabapentin use in pregnancy are complex, it’s important to talk with your doctor about whether starting or continuing use is right Gabapentin/first trimester of pregnancy: 10 newborns (0.01%) [30] Patorno et al., 2020: Gabapentin/first trimester of pregnancy: 4642 pregnancies (0.26%) Patorno et al., 2020: Gabapentin/early in pregnancy (first 140 days of pregnancy and no gabapentin dispensing from the 141 and 245 days)3745 pregnancies (0.21%) Patorno et al., 2020 Despite the widespread use, only sparse information is available on the safety of gabapentin during pregnancy. We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes. Advice and warnings for the use of Gabapentin during pregnancy. FDA Pregnancy Category C - Risk cannot be ruled out Does taking gabapentin increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Small, controlled studies on gabapentin have not suggested an increased chance of birth defects. The majority of the available epidemiological data relate to gabapentin use in pregnancy for the treatment of maternal epilepsy. A few case reports/series describe use of gabapentin in the treatment of neuropathic pain or hyperemesis gravidarum but studies have not assessed fetal outcomes following use in pregnancy for these indications. It is not known if gabapentin can make it harder to get pregnant. Sexual dysfunction (including loss of desire to have sex and loss of ability to have an orgasm) has been reported among women who take gabapentin. Taken together, the current literature suggests that gabapentin use should be considered with caution during pregnancy and during the post-partum period. Well-controlled, prospective research studies are needed to determine the extent of the risks and benefits of prescribed and nonprescribed gabapentin exposure to pregnant people and their We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. There was an increased risk of preterm birth among women exposed to gabapentin either late (RR=1.28 [CI 1.08-1.52], p < 0.01) or both early and late in pregnancy (RR=1.22 [1.09-1.36], p < 0.001). Selected References: Blotiere PO, et al. 2020. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open 10(6). Brannon GE, Rolland PD. Anorgasmia in a patient with bipolar disorder type 1 treated with gabapentin. J Clin Psychopharmacol. 2000;20(3):379
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |