Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
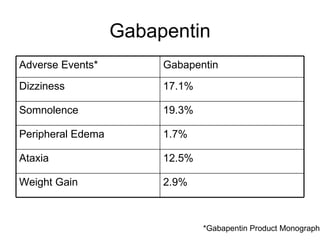 | 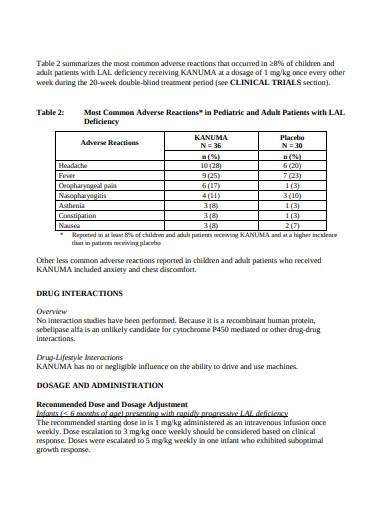 |
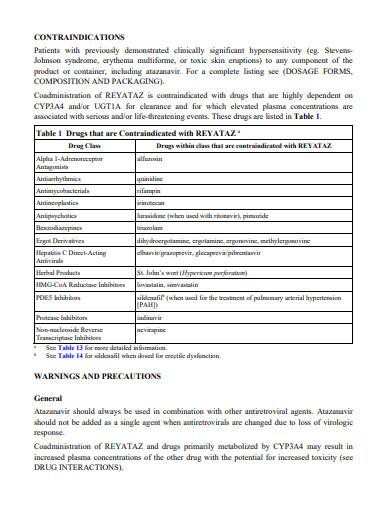
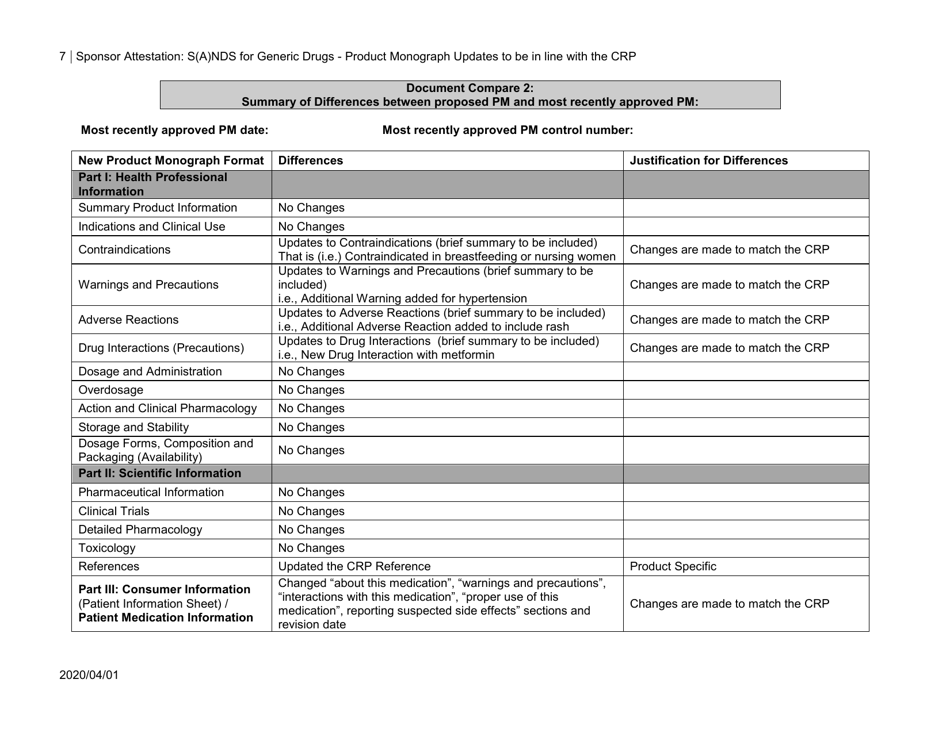
GABAPENTIN (Gabapentin Tablets) Page 1 of 37 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrGABAPENTIN Gabapentin Tablets Tablets, 600 mg and 800 mg, Oral USP Antiepileptic Agent JAMP Pharma Corporation 1310 rue Nobel Boucherville, Quebec NEURONTIN (gabapentin) Page 1 of 36 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrNEURONTIN® Gabapentin Capsules Capsules, 100 mg, 300 mg and 400 mg, oral Gabapentin Tablets Tablets, 600 mg and 800 mg, oral Antiepileptic Agent BGP Pharma ULC 85 Advance Road Etobicoke (Ontario) Canada M8Z 2S6 Submission Control No.: 274395 The product monograph is developed by a drug sponsor according to guidelines published by Health Canada that provide direction on the content and format. The veterinary labelling is developed by the drug sponsor according to the Food and Drug Regulations. Search the Drug Product Database (DPD) to find drugs authorized for sale by Health Canada. The DPD is updated nightly and includes: availability of the drug in Canada ; product monograph (PM) for human drugs ; labels for animal drugs; Generic drug manufacturers must update their PM to ensure it aligns with the Canadian Reference Product. NEURONTIN® (gabapentin) Product Monograph Page 1 of 29 PRODUCT MONOGRAPH Pr NEURONTIN® Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Pfizer Canada Inc. 17, 300 Trans-Canada Highway Kirkland, Quebec H9J 2M5 www.pfizer.ca Date of Revision: February 22, 2018 Submission Control No: 211678 TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Geriatrics (>65 years of age) This leaflet is part III of a three-part "Product Monograph" published when GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Gabapentin Page 1 of 28 PRODUCT MONOGRAPH Pr Gabapentin Gabapentin Tablets, House Standard 600 mg and 800 mg Antiepileptic Agent JAMP Pharma Corporation 1310 rue Nobel Boucherville, Quebec J4B 5H3 Control# 256895 GABAPENTIN Product Monograph Page 3 of 32 Pr GABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg, and 400 mg PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Dosage Form / Strength All Non-medicinal Ingredients Oral Capsules / 100 mg, 300 mg, and 400 mg Corn Starch, Lactose Anhydrous, and Talc. PRODUCT MONOGRAPH PrNEURONTIN® Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent May 5, 2020 Date of Revision: ©Upjohn Canada ULC,2020 Upjohn Canada ULC, Licensee ®Warner-Lambert CompanyLLC Submission Control No.: 237475 H9J 2M5 Kirkland, Quebec 17300Trans-Canada Highway UpjohnCanadaULC Product Monograph: The Product Monograph is a scientific document that describes the properties, claims, indications and conditions of use of the product and contains any other information that may be required for optimal, safe and effective use. The Product Monograph includes three sections: Part I: Health Professional Information; GABAPENTIN Product Monograph Page 3 of 32 PrGABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg and 400 mg PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Dosage Form / Strength All Non-medicinal Ingredients Oral Capsules: 100 mg, 300 mg, and 400 mg Corn Starch, Lactose Anhydrous, and Talc. NEURONTIN® (gabapentin) Product Monograph Page 17 of 29 ACTION AND CLINICAL PHARMACOLOGY Mechanism of Action Neurontin (gabapentin) readily enters the brain and prevents seizures in a number of animal models of epilepsy. PRODUCT MONOGRAPH Pr MYLAN-GABAPENTIN (Gabapentin Capsules) 100 mg, 300 mg, and 400 mg (Gabapentin Tablets, USP) 600 mg and 800 mg ANTIEPILEPTIC AGENT NEURONTIN (gabapentin) PART III: CONSUMER INFORMATION. Pr. NEURONTIN® (gabapentin) This leaflet is part III of a three-part “Product Monograph" published when NEURONTIN was approved for sale in Canada and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about NEURONTIN. Contact your doctor or Information on drug and health products authorized by Health Canada. while taking GABAPENTIN, contact your doctor or pharmacist. product monograph that is Neurontin (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). Product name: APO-GABAPENTIN Company name: APOTEX INC DIN: 02293358 Status: Marketed Status date: 2020-07-15
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |