Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
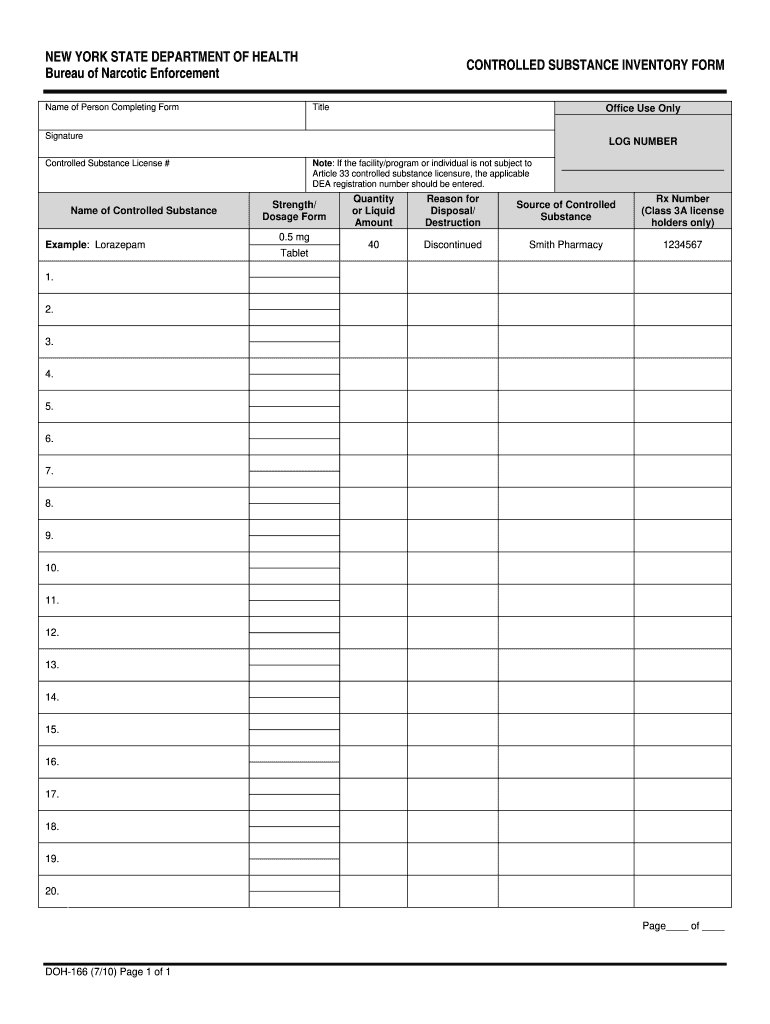
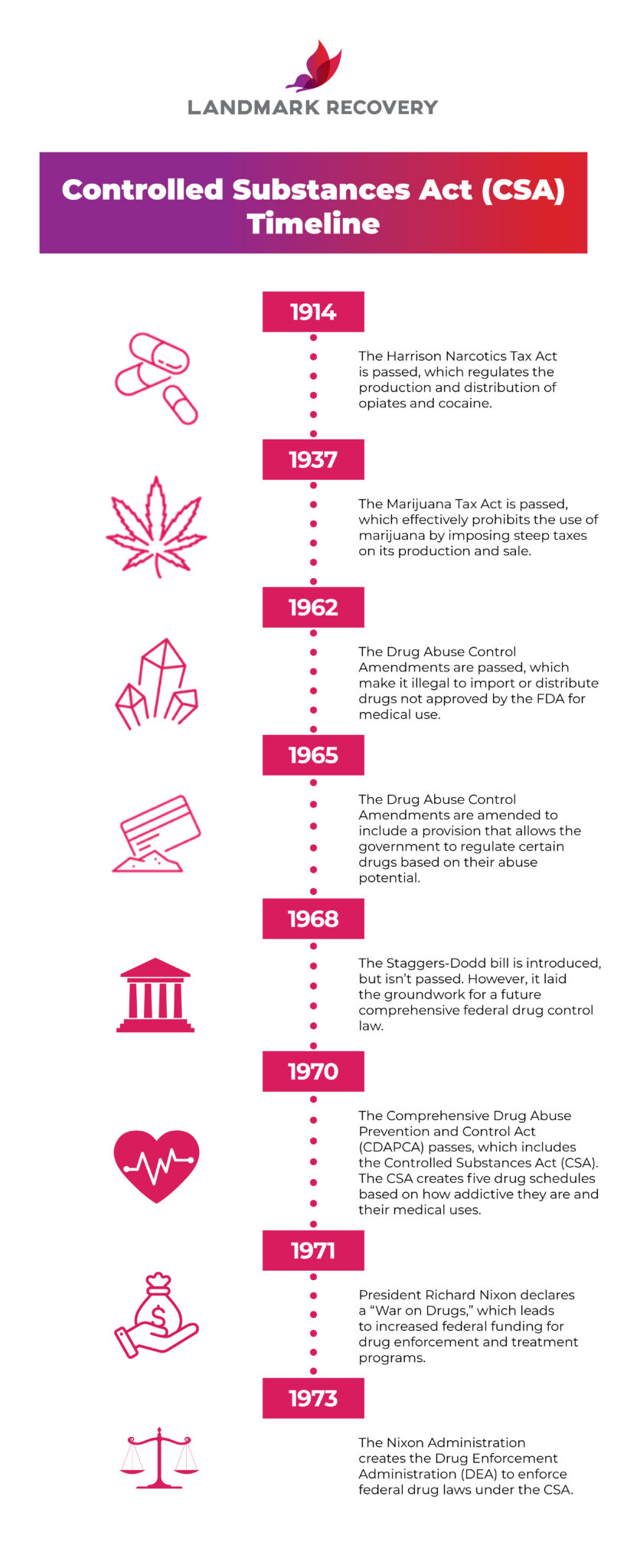
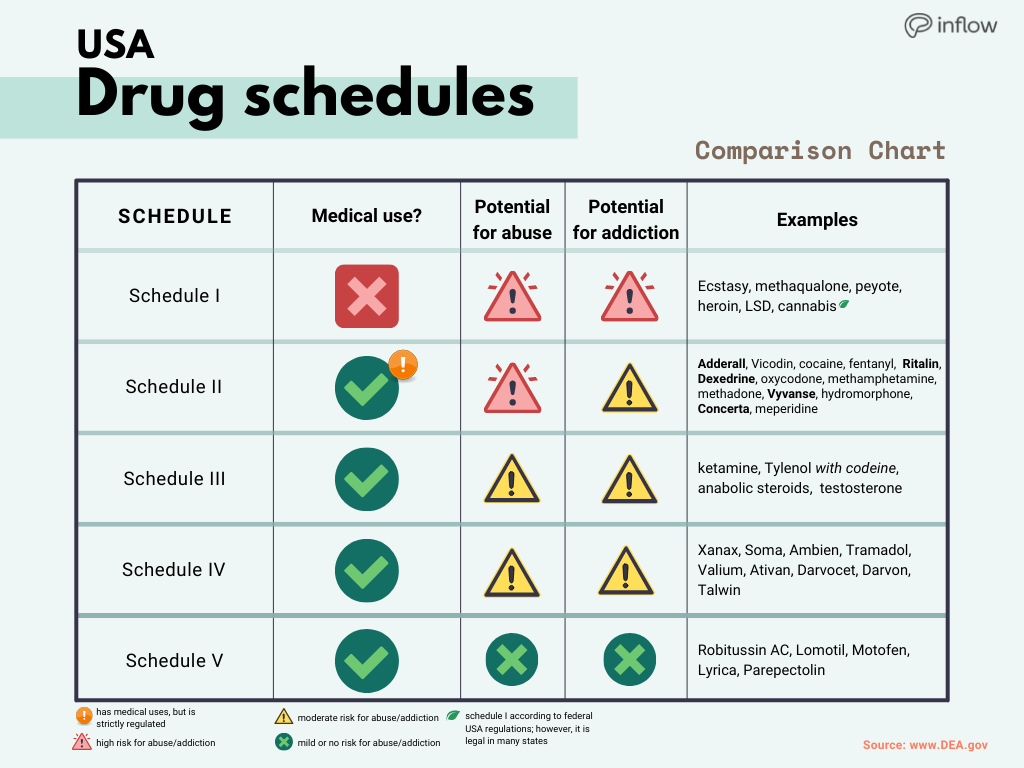
Controlled substances: schedules; classification of gabapentin as a schedule 5 controlled substance; modify. Amends sec. 7201 of 1978 PA 368 (MCL 333.7201). SUBSTANCE CSCN CSA SCH NARC OTHER NAMES 1-Androstenedione (5α-androst-1-en-3,17-dione) 4000 III N 1-Methyl-4-phenyl-4-propionoxypiperidine 9661 I Y MPPP, synthetic heroin January 9, 2019 – In an effort to continue to combat the opioid epidemic in Michigan, the Dept. of Licensing and Regulatory Affairs (LARA), with the support of the Michigan Board of Pharmacy, has modified its Pharmacy Rules to categorize Gabapentin as a Schedule 5 controlled substance. Gabapentin – or Neurontin – is a medication commonly At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73(b) adds it to the medications recorded in NC CSRS because it may cause a level of sedation in patients that puts them at increased risk of overdose when taken with opioids. The NC Department of Health and Human Services (NCDHHS) has , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. In an effort to continue to combat the opioid epidemic in Michigan, the Dept. of Licensing and Regulatory Affairs (LARA), with the support of the Michigan Board of Pharmacy, has modified its Pharmacy Rules to categorize Gabapentin as a Schedule 5 controlled substance. Gabapentin – or Neurontin – is a medication commonly used to treat nerve Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. A prescriber without a controlled substance license can now prescribe Gabapentin, and Gabapentin no longer needs to be included in reporting to the Michigan Automated Prescription System (MAPS). Despite this, we encourage prescribers and dispensers to continue to monitor the use and potential misuse of this drug. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Michigan prescribers and patients have experienced the implementation of several legislative and regulatory actions intended to address Michigan's opioid crisis over the past year. Most recently, revisions to the Michigan Board of Pharmacy's Controlled Substance rules were finalized and took effect immediately upon filing with the Office of the Great Seal on January 4, 2019. There were three in the scheduling of controlled substances: • Gabapentin will be removed from Schedule 5 and descheduled. • Pentazocine will be rescheduled from a Schedule 5 drug to a Schedule 4 drug. PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was Drugs and other substances that are considered controlled substances under the Controlled Substances Act (CSA) are divided into five schedules. An updated and complete list of the schedules is published annually in Title 21 Code of Federal Regulations (C.F.R.) §§1308.11 through 1308.15. Substances are placed in their respective schedules Michigan is listing a drug commonly used to treat nerve pain and seizures as a controlled substance, a step intended to fight the opioid epidemic. 1 weather alerts 1 closings/delays Watch Now Although the anticonvulsant is not considered a controlled substance, some state legislation focuses on monitoring the use of or reclassifying it. The FDA approved gabapentin in 1993 as a non-controlled substance and it has remained a non-controlled substance at the federal level. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The number of states placing gabapentin on the controlled substance list or in their required monitoring program is growing and three more states are debating whether to add gabapentin as a controlled substance or to their mandated reporting programs (DE, NY, and WI).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |