Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
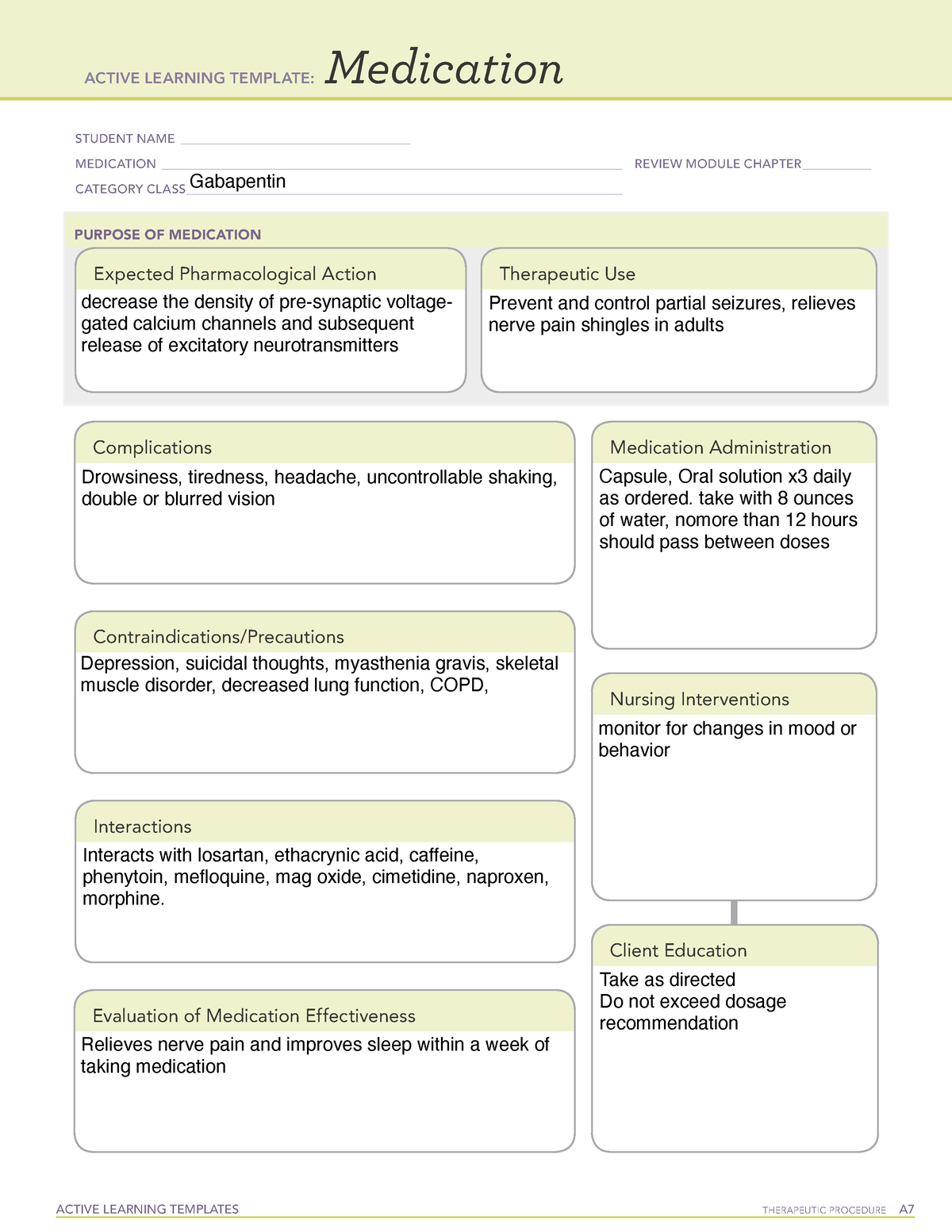
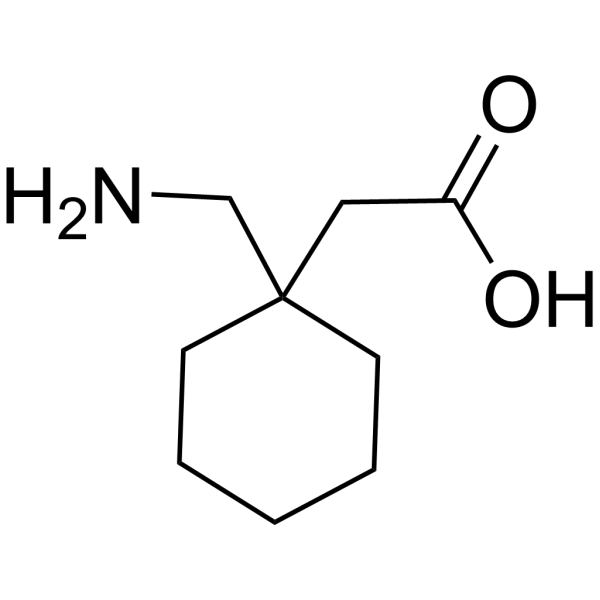
SAFETY DATA SHEET Section 1: Identification Material Recommended use Gabapentin Capsules USP 100 mg, 300 mg and 400 mg Pharmaceutical product used as anticonvulsant. Manufacturer Hetero Labs Limited, Plot No. 28P1 to 36P1 and 37 to 54, Vemagal Industries Area, Hobli Vemagal Kolar - 563102, Karnataka, India. Product Name: Gabapentin : Cat. Number: S2133: Manufacturer/Supplier: Selleck Chemicals 14408 W Sylvanfield Drive, Houston, TX 77014 USA Toll Free:(877) 796-6397 (US and Canada only) Tel:+1-832-582-8158 Fax:+1-832-582-8590 Material Name: Gabapentin Capsules (100 mg, 300 mg and 400 mg) Trade Name: NEURONTIN; NEUGABA; GANTIN; PARKETIN; GABAPENTIN PFIZER Chemical Family: Mixture Relevant Identified Uses of the Substance or Mixture and Uses Advised Against Intended Use: Pharmaceutical product used as anticonvulsant Details of the Supplier of the Safety Data Sheet 2. Material Name: Neurontin (gabapentin) Oral Solution Trade Name: NEURONTIN Chemical Family: Mixture Relevant Identified Uses of the Substance or Mixture and Uses Advised Against Intended Use: Pharmaceutical product used as anticonvulsant Details of the Supplier of the Safety Data Sheet 2. HAZARDS IDENTIFICATION Classification of the Substance or Chemical Safety Data Sheet MSDS / SDS Gabapentin Revision Date:2025-03-08 Revision Number:1 SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifier Relevant identified uses of the substance or mixture and uses advised against Company Identification SECTION 2: Hazards identification SAFETY DATA SHEET ----- Date: 06/29/2015 Generic Name: Gabapentin Capsules, USP 100, 300 and 400 mg. Brand Equivalent: Neurontin® (Gabapentin) Capsules, 100/300 and 400 mg ----- SECTION 1: IDENTIFICATION Product Name Gabapentin Capsules, USP Active substance Gabapentin Synonyms N/A Formula C 9 H 17 NO 2 MATERIAL SAFETY DATA SHEET _____ Material Name: Neurontin (gabapentin) Oral Solution Revision date: 02-Jan-2007 Page 2 of 6 Version: 1.3 Note: This document has been prepared in accordance with standards for workplace safety, which require the inclusion of all known hazards of the product or its ingredients regardless of the potential risk. SAFETY DATA SHEET Gabapentin Capsules, USP 1. IDENTIFICATION Manufacturer: Emergency Phone: Ascent Pharmaceuticals Inc 1-855-221-1622 400 S.Technology Dr. Material Safety Data Sheet . Material Name: Gabapentin Oral Solution, 250 mg / 5 mL _____ Page 2 of 5 Issue Date: 02/16/12 Revision: 1.0000 Print Date: 2/16/2012 . First Aid: Inhalation. Remove the person from the exposed area to fresh air immediately. GABAPENTIN EEC No. 262-076-3 Not available60142-96-3 Proprietary Excipients - Not available - Remainder SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product Name Page 1 / 6 Gabapentin Tablets and Capsules Revision Date 09-Mar-2017 Active Ingredient Gabapentin Safety Data Sheet acc. to OSHA HCS Printing date 02/21/2024 Revision date 02/21/2024 58.0.12 * 1 Identification · Product identifier · Trade name:Gabapentin-d4 · Synonym1-(aminomethyl-d2)-cyclohexaneacetic-α,α-d2 acid · Article number:18254 · CAS Number: 1185039-20-6 · Application of the substance / the mixture JIS Z 7253: 2019. The information provided in this Safety Data Sheet is correct to the best of our knowledge, information and belief at the date o. its publication. The information given is designed only as a guidance for safe handling, and is not to be considered a warranty or qual. SAFETY DATA SHEET Gabapentin Tablets, USP 1. IDENTIFICATION Manufacturer: Emergency Phone: InvaGen Pharmaceuticals Inc 1-631-231-3233 7, Oser Avenue Hauppauge, NY MATERIAL SAFETY DATA SHEET Version: 1.2 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Material Name: Gabapentin Capsules (100 mg, 300 mg and 400 mg) Trade Name: Neurontin® Chemical Family: Mixture Intended Use: Pharmaceutical product used as anticonvulsant 2. COMPOSITION/INFORMATION ON INGREDIENTS Hazardous SAFETY DATA SHEET Gabapentin Capsules, USP 1. IDENTIFICATION Manufacturer: Emergency Phone: Ascent Pharmaceuticals Inc 1-855-221-1622 550 S.Research Place MATERIAL SAFETY DATA SHEET _____ 8. EXPOSURE CONTROLS / PERSONAL PROTECTION The exposure limit(s) listed for solid components are only relevant if dust may be generated. Analytical Method: Analytical method available for gabapentin. Contact Pfizer Inc for further information. Show this safety data sheet to the doctor in attendance. Immediate medical attention is required. Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Gabapentin pre vents pain-related responses in several models of neuropathic pain in rats and mice (e.g., spinal nerve ligation models, spinal cord injury model, acute herpes zoster infection model). Safety Data Sheet acc. to OSHA HCS Printing date 12/05/2023 Revision date 12/05/2023 57.0.42 * 1 Identification · Product identifier · Trade name:Gabapentin · Synonym CI-945 1-(aminomethyl)-cyclohexaneacetic acid · Article number:10008346 · CAS Number: 60142-96-3 · EC number: 262-076-3 · Application of the substance / the mixture Common Name: Gabapentin Capsules, USP . Synonym(s): Neurontin Chemical Name: 1-(Aminomethyl)cyclohexaneacetic Acid . Chemical Family: Cyclohexane-acetic acid derivative . Trade Name(s): Gabapentin Capsules, USP 100 mg, 300 mg and 400 mg. Therapeutic Category: Used. to relieve pain, especially neuropathic pain; Anticonvulsant. Molecular formula
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |