Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
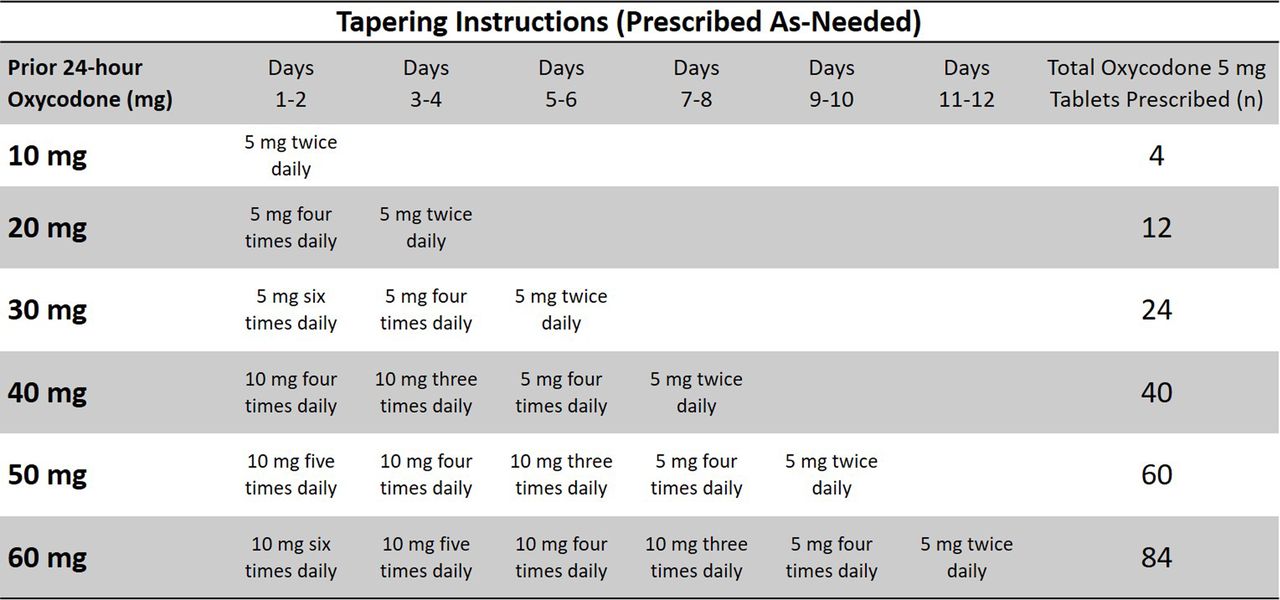
A gabapentin taper chart can provide structure, helping you gradually reduce your dose while minimizing the discomfort that can come with stopping too quickly. It’s not about rushing—it’s about finding a steady, safe way forward that works for you. We would like to show you a description here but the site won’t allow us. Gabapentin (Neurontin) is an antiseizure medication. It’s also used for nerve pain from shingles. Other long-acting forms called Gralise and Horizant are also available. For adults, your gabapentin dosage varies depending on your medical conditions and which form you’re taking. The maximum dosage is 3,600 mg per day. Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote. • Gabapentin: Policymakers are increasingly interested in monitoring Gabapentin due to a recent uptick in Gabapentin prescriptions and its regular involvement in overdoses. As a drug that can curb opioid withdrawals and lessen the effects of medications used for addiction treatment, Gabapentin is widely misused. Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Af ter May 1 , 2024, any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. Schedule I controlled substances. This schedule includes the controlled substances listed or to be listed by whatever official name, common or usual name, chemical name, or trade name designated. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. 37-2707. Schedule II. (a) Schedule II shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Gabapentin is chemically known as 2-[1-(aminomethyl) cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. (ii) Any compound containing a 1H-indol-3-yl-(1-naphthyl)methane structure, also known as napthylmethylindoles, with substitution at the nitrogen atom of the indole ring by an alkyl, haloalkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl, 1-(N-methyl-2-piperidinyl)methyl, or 2-(4-morpholinyl)ethyl group, whether or not further substituted on the indole ring to any extent and whether or not MHRA/CHM advice: Gabapentin (Neurontin®) and risk of abuse and dependence: new scheduling requirements from 1 April (April 2019) Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Day 1: 300 mg orally once Day 2: 300 mg orally 2 times day Day 3: 300 mg orally 3 times a day. Titrate dose as needed for pain relief; Maintenance dose: 900 to 1800 mg/day orally in 3 divided doses Maximum dose: 1800 mg per day Extended-release: Gralise (gabapentin) 24-hour extended-release tablets: Initial dose: Day 1: 300 mg orally with the than 1 in 5 people (22%) who had a drug overdose-related death were positive for gabapentin. Furthermore, approxi-mately 1 in 4 people (26%) who tested positive for opiates also testedpositiveforgabapentin.Onthebasisof thisanalysis,the prevalence of gabapentin abuse may have been previously underestimated in the population.25 PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. See the notes in regards to combination products. Always verify the schedule of combination products. Contact pharmacist if there is a question.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |