Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |
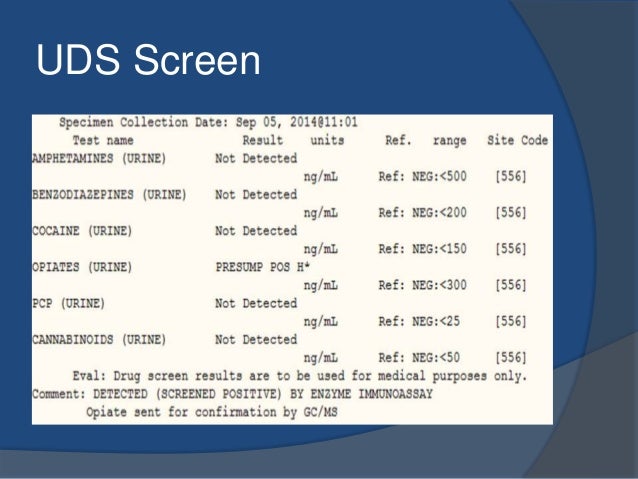
But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Talk to your healthcare provider about the risks of gabapentin before taking it. Neurontin (gabapentin) is used to treat pain you may have from shingles (postherpetic nerve pain). It is also used with other seizure medicines for partial onset seizures in patients 3 years and older. Gralise (gabapentin) is only used for pain after having shingles (postherpetic nerve pain). It should not be used for any other medical condition. Appendix D of the Poisons and Therapeutic Goods Regulation 2008 (Regulation) lists Schedule 4 substances (prescription-only medicines) that have common therapeutic uses, but are also liable to abuse, misuse and diversion, warranting more stringent controls on possession and supply. A gabapentin taper chart can provide structure, helping you gradually reduce your dose while minimizing the discomfort that can come with stopping too quickly. It’s not about rushing—it’s about finding a steady, safe way forward that works for you. Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. Is Gabapentin a Schedule 4 drug? Yes, gabapentin is classified as a Schedule V controlled substance in some states, while it is controlled federally in all states due to its similarity to pregabalin, which is a Schedule V drug. Examples of Schedule 3 CDs include tramadol, gabapentin, and pregabalin. Schedule 3 CDs are exempt from safe custody requirements, however, the RCVS advises that all Schedule 3 CDs should be stored in a CD cabinet. CDs in Schedule 4 are divided into two parts. Gabapentin enacarbil available under the trade name Horizant is the only gabapentin product approved for treatment of Restless Legs Syndrome (RLS). A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose, but caused an increase in adverse reactions. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Schedule IV drugs, substances, or chemicals are defined as drugs with a low potential for abuse and low risk of dependence. Some examples of Schedule IV drugs are: Xanax, Soma, Darvon, Darvocet, Valium, Ativan, Talwin, Ambien, Tramadol. While gabapentin is not a controlled substance, rule 4729:8-2-02 requires the following entities to submit the specified dispensing, personal furnishing, or wholesale sale information on all products containing gabapentin to the Ohio Automated Rx Reporting System (OARRS): Practical Impact for Many Prescribers and Dispensers of Gabapentin Because Gabapentin has been designated a monitored prescription drug, a practitioner now must review a patient’s prescription drug history as required by Wis. Admin. Code § CSB 4.105 prior to prescribing Gabapentin. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. Appendix B of the Poisons and Therapeutic Goods Regulation 2008 (Regulation) lists Schedule 4 substances (prescription-only medicines) that have more stringent controls on possession and supply because they are liable to abuse, misuse and diversion. These substances are referred to under the Regulation as special restricted substanc because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine. open tool tip to find out more. 100s pack; Pregnant or planning a pregnancy ? For the active ingredient gabapentin. You should seek advice from your doctor or pharmacist about taking this medicine. Veterinary surgeons must store CDs securely and appropriately in a suitable cabinet to prevent unauthorised access. All Schedule 2 CDs, with the exception of quinalbarbitone, as well as Schedule 3 CDs containing buprenorphine, diethylpropion, flunitrazepam, and temazepam, are legally required to be stored in a locked cabinet which is compliant with the Safe Custody Regulations. purposes of this Schedule I hallucinogenic substances section only, the term “isomer” includes optical, position, and geometric isomers): (1) α-Ethyltryptamine (Other names: etryptamine; Monase; α-
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |