Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
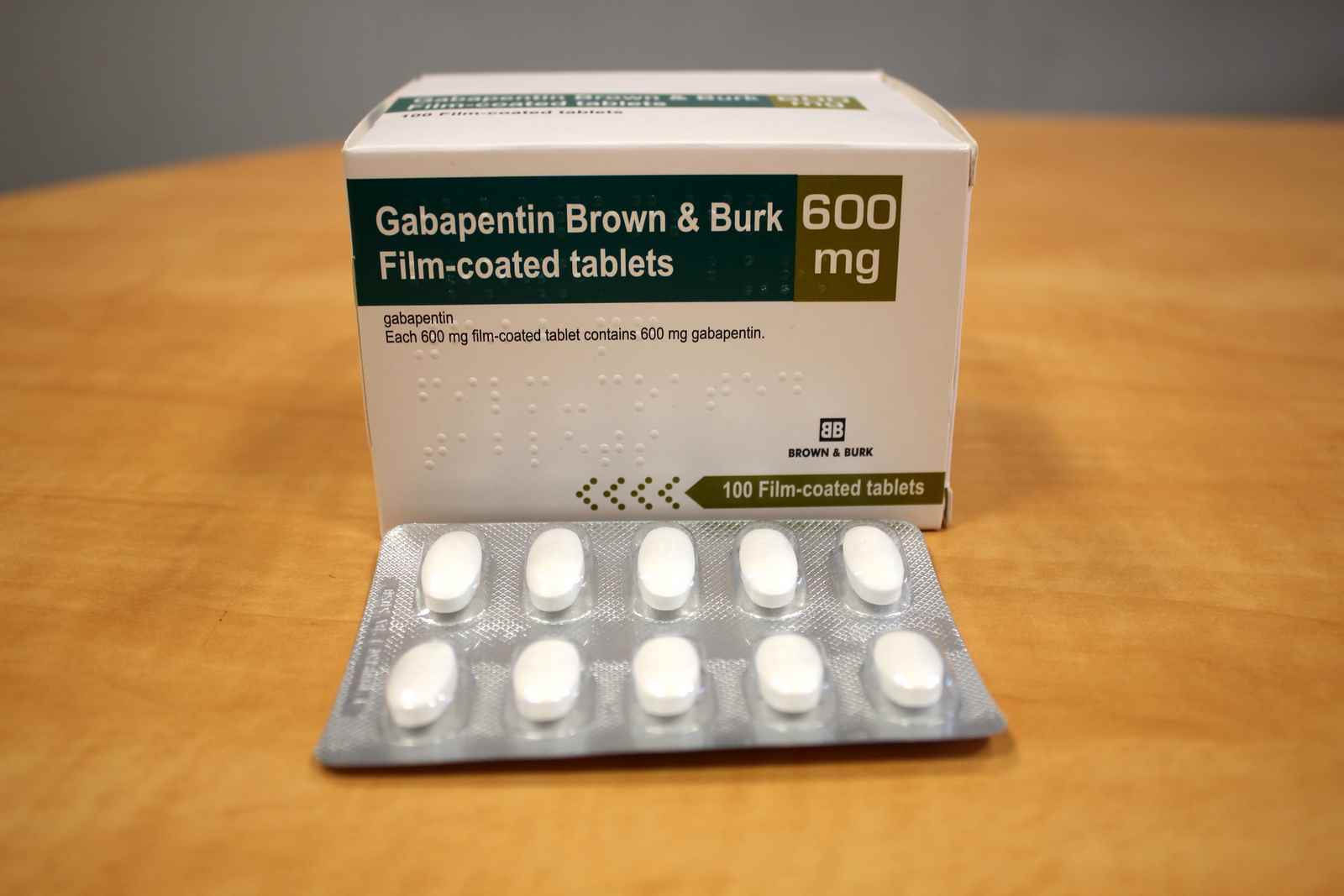
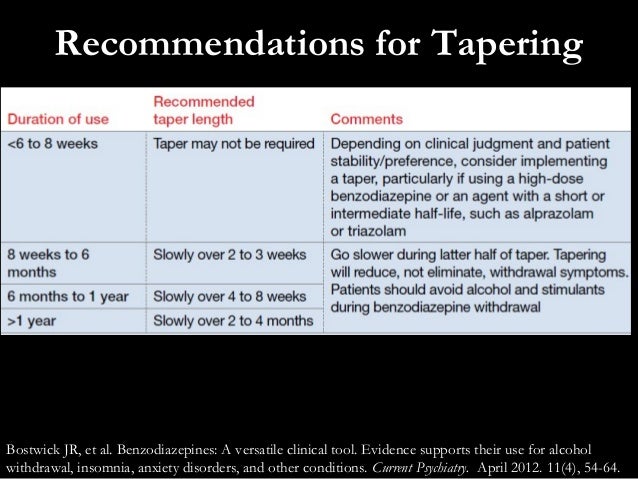
In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). By: Heidi Dufrene, PharmD . Recently, the Alabama State Committee of Public Health voted to change Gabapentin to a Schedule V medication. This ruling will become effective November 18, 2019, giving pharmacies and other health care providers adequate time to implement necessary changes. What states consider gabapentin a controlled substance? As of July 2022, these states consider gabapentin a schedule V controlled substance: Alabama. Kentucky. Michigan. North Dakota. Tennessee. Virginia. West Virginia. Other states have mandated gabapentin reporting. Every time you fill a gabapentin prescription, it’s added to the PDMP (Adopted by Alabama State Board of Health on February 21, 2018, effective February 21, 2018) Schedule I (a) Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, or brand name designated, listed in this section. Each drug or substance (Adopted by Alabama State Board of Health on January 15, 2020, effective January 15, 2020) Schedule I (a) Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, or brand name designated, listed in this section. Each drug or substance Effective November 18, 2019, Gabapentin is a Schedule V Controlled Substance in Alabama. Though Gabapentin is not a controlled substance at the federal level, Alabama joins a growing list of states that have reclassified the drug as a controlled substance in the past year. Recently, the Alabama State Committee of Public Health voted to change Gabapentin to a Schedule V medication. This ruling will become effective November 18, 2019, giving pharmacies and other health care providers adequate time to implement necessary changes. (a) Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, or brand name designated, listed in this section. Each drug or substance has been assigned the DEA Controlled Substances Code Number set forth opposite it. (b) Opiates. (Adopted by Alabama State Board of Health on January 20, 2022, effective January 20, 2022) Schedule I (a) Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, or brand name designated, listed in this section. Each drug or substance Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. After adjustment, Schedule V gabapentin regulation resulted in a reduction of 8.37 total days of gabapentin prescribed per enrollee (95% confidence interval of - 10.34 to - 6.39). In contrast, PDMP regulation resulted in a reduction of 1.01 total days of gabapentin prescribed per enrollee (95% confidence interval of - 1.74 to - 0.29). Drugs 37 4-Hydroxytestosterone (4,17beta-dihydroxyandrost-4-en-3-on 4000 III N 38 4-Methoxyamphetamine 7411 I N PMA 39 4-Methyl-2,5-dimethoxyamphetamine 7395 I N DOM, STP Of those cases, gabapentin was the primary cause of death in 23 individuals. According to this report, gabapentin was amongst one of the . Of the 154 analytes detected, gabapentin accounted for 25 samples. Total exposure calls as a result of gabapentin stayed largely the same between 2017 and in 2017 and 21,423 in 2020. Among cases Controlled substances (CS) are regulated by the federal Controlled Substances Act (CSA), which divides CS into five categories called schedules. Schedule I drugs have a high potential for abuse and currently have no accepted medical use. They are the only schedule of drugs that cannot be prescribed. Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, or brand name designated, listed in this section. Each drug or substance has been assigned the DEA Controlled Substances Code Number set forth opposite it. Opiates. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Schedule I - Listing of controlled substances. (a) The Legislature finds the following: (1) New synthetic substances are being created which are not controlled under the provisions of existing state law but which have a potential for abuse similar to or greater than that for substances controlled under existing state law. Gabapentin isn't a narcotic, but it is a controlled substance in some states. labeling gabapentin as a Schedule 5 controlled substance. Alabama; Kentucky; Michigan; North Dakota; Tennessee Ohio 12/1/2016 PDMP Reporting for Gabapentin Tennessee 7/1/2018 Schedule V Classification of Gabapentin Virginia 2/23/2017 PDMP Reporting for Gabapentin West Virginia 6/7/2018 Schedule V Classification of Gabapentin Wyoming 7/1/2017 PDMP Reporting for Gabapentin JGIM Grauer and Cramer: Association of State-Imposed Restrictions on Gabapentin 3631
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |