Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
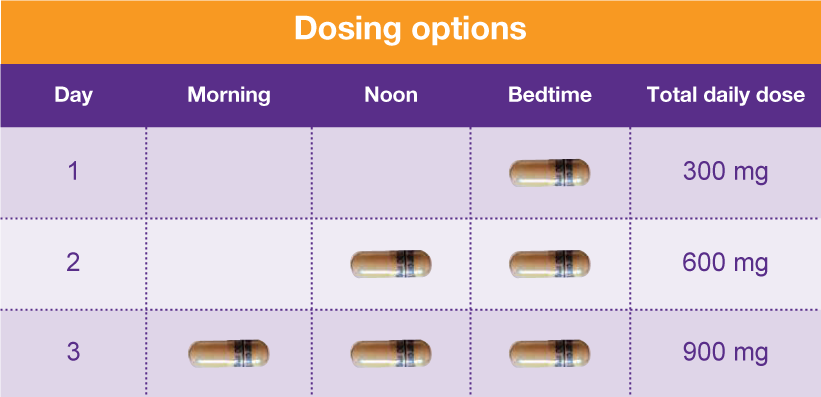
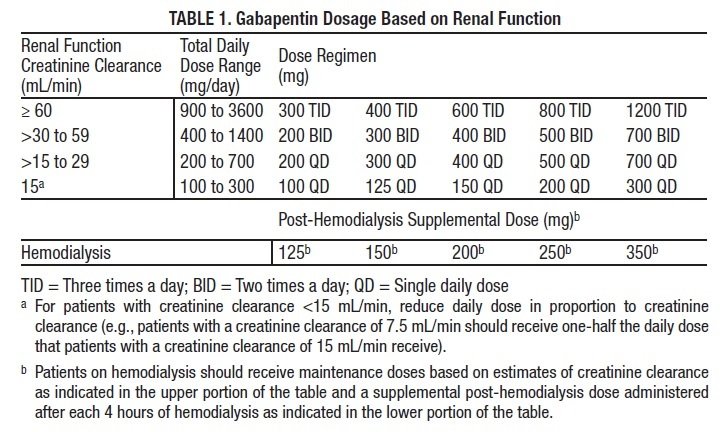
States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Table 1 Summary of Gabapentin Policy Changes 2013–2018 State Policy start date Gabapentin policy type Kansas 7/25/2017 PDMP Reporting for Gabapentin Kentucky 3/3/2017 Schedule V Classification of Gabapentin Massachusetts 8/1/2017 PDMP Reporting for Gabapentin Minnesota 8/1/2016 PDMP Reporting for Gabapentin Nebraska 1/1/2018 PDMP Reporting Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Schedule-V controlled substance and mandated reporting to PDMP. The State of Kentucky is, and to date, remains, the only state to have reclassified gabapentin as a Schedule-V controlled substance. 21 Effective July 1, 2017, the prescribing of gabapentin is limited to authorized practitioners, defined as practitioners registered with the US DEA. 21 Thus, mid-level practitioners, specifically Section 1. Schedule I Controlled Substances. (1) Each substance that is scheduled or designated as a Schedule I controlled substance under 21 C.F.R. 1308.11, including a substance temporarily scheduled or designated under 21 C.F.R. 1308.11(h) or 1308.49, shall be scheduled or designated at the state level as a Schedule I controlled substance. Our data support that this policy is effective at lowering rates of gabapentin prescribing in the Medicare Part D enrollee population. To support the national imperative to reduce gabapentin prescribing, the FDA may consider changing the federal gabapentin schedule classification to Schedule V. Limitations Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. (a) Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Each drug or substance has been assigned the DEA Controlled Substances Code Number set forth opposite it. FDA announced a new mandate that labels of gabapentin and pregabalin contain a warning about respiratory depression . ii Pregabalin is controlled in Schedule V of the Federal Controlled Substances Act . Although not controlled federally, some States also list gabapentin as a Schedule V controlled • Gabapentin: Policymakers are increasingly interested in monitoring Gabapentin due to a recent uptick in Gabapentin prescriptions and its regular involvement in overdoses. As a drug that can curb opioid withdrawals and lessen the effects of medications used for addiction treatment, Gabapentin is widely misused. For schedules, the rule changes adopt the federal schedule subject to drugs scheduled by the state after January 6, 2022, and the rules promulgated by the Michigan Board of Pharmacy; remove Brorphine, Gabapentin, and Pentazocine as exceptions to the federal schedule; provide an exception to the federal scheduling for isomers, Salvia Divorum Background Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear. Objective To determine the effect of state-imposed “The effort to put gabapentin on the federal Schedule 5 list may reduce its use for pain syndromes where it could actually help, thereby leading to increased use of other agents that have more side effects and drug interactions and possibly even translate to greater use of agents that have pronounced addictive potential such as tramadol and Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |