Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 | 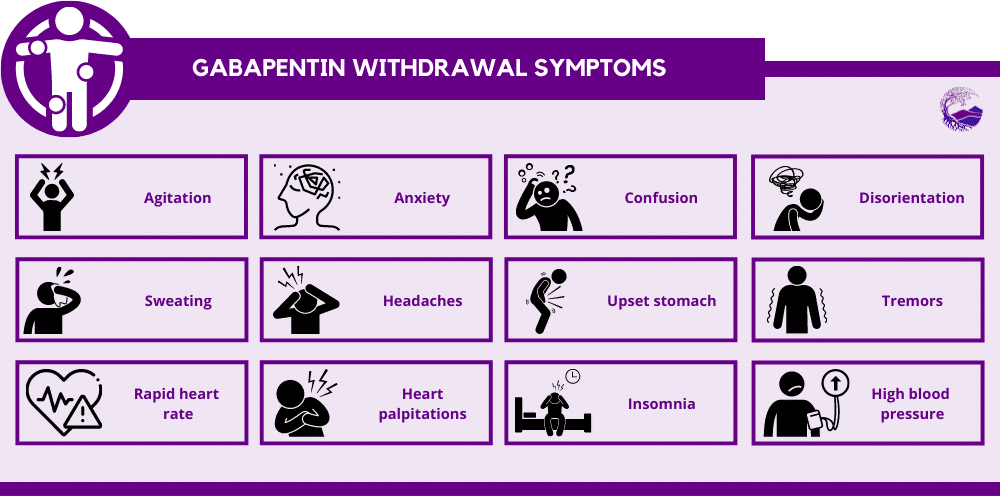 |
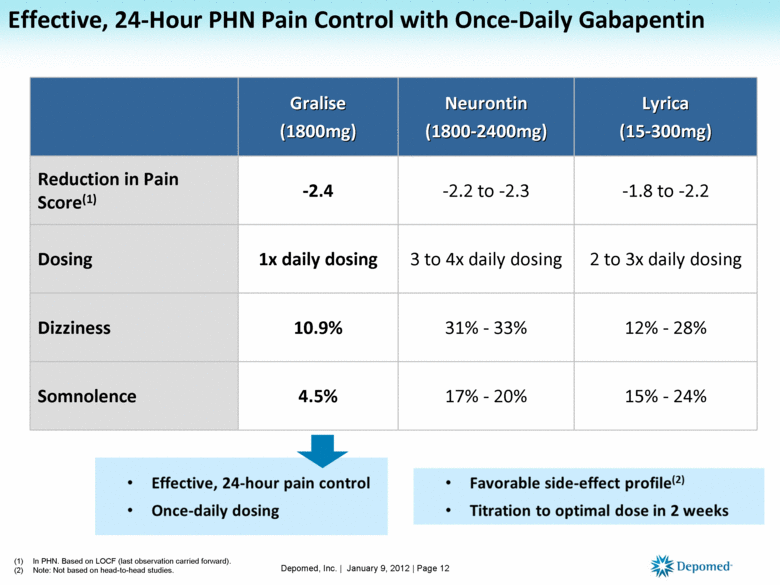
Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). (1) SCHEDULE I. — A substance in Schedule I has a high potential for abuse and has no currently accepted medical use in treatment in the United States and in its use under medical supervision does not meet accepted safety standards. The following substances are controlled in Schedule I: Gabapentin has been a federally noncontrolled substance since its FDA approval in 1993. It’s typically used for epilepsy and nerve pain, a severe symptom that other prescription medications can Chronic pain and opioid use and abuse is a significant problem in the United States and in Florida.[1] Over one-quarter of United States citizens suffer from chronic pain.[2] It is among the most common complaints seen in an outpatient clinic and the emergency department. The failure to manage chronic pain, as well as the possible complication of opioid dependence related to treatment, can Schedule II drugs: hydromorphone (Dilaudid), methadone (Dolophine), meperidine (Demerol), oxycodone (OxyContin, Percocet), and fentanyl (Sublimaze, Duragesic). Other Schedule II narcotics include 64. Nitazene derivatives. Unless specifically excepted, listed in another schedule, or contained within a pharmaceutical product approved by the United States Food and Drug Administration, any material, compound, mixture, or preparation, including its salts, isomers, esters, or ethers, and salts of isomers, esters, or ethers, whenever the existence of such salts is possible within any of the Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The MQA’s FY 2024-2025 Quarter One Performance Report is Out! February 3, 2025. Announcing MQA’s FY 2024-2025 Performance Report for Quarter One Continue reading → (1) SCHEDULE I.— A substance in Schedule I has a high potential for abuse and has no currently accepted medical use in treatment in the United States and in its use under medical supervision does not meet accepted safety standards. The following substances are controlled in Schedule I: 1. Acetyl-alpha-methylfentanyl. 2. Acetylmethadol. 3. Still Detox in Boca Raton, Florida provides specialized programs for individuals facing gabapentin dependence, including a structured gabapentin taper schedule. Call Now: (561) 556-2677 Background Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear. Objective To determine the effect of state-imposed Of those cases, gabapentin was the primary cause of death in 23 individuals. According to this report, gabapentin was amongst one of the . Of the 154 analytes detected, gabapentin accounted for 25 samples. Total exposure calls as a result of gabapentin stayed largely the same between 2017 and in 2017 and 21,423 in 2020. Among cases Gabapentin Abuse Tuyet-Anh Nguyen itself is not a controlled substance in Florida, it is a schedule 5 in Kentucky, Virginia, West Virginia, Tennessee, and E-FORCSE®: Florida’s Prescription Drug Monitoring Program Florida’s Prescription Drug Monitoring Program, known as E-FORCSE® (Electronic-Florida Online Reporting of Controlled Substances Evaluation) is a database that collects and stores schedule II-V controlled substance dispensing information.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |