Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
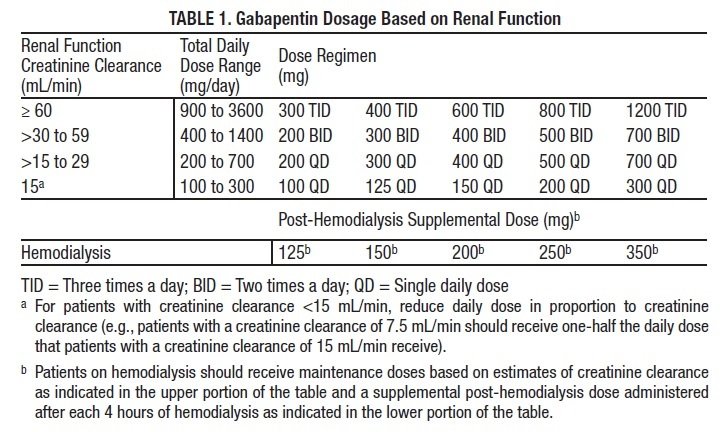
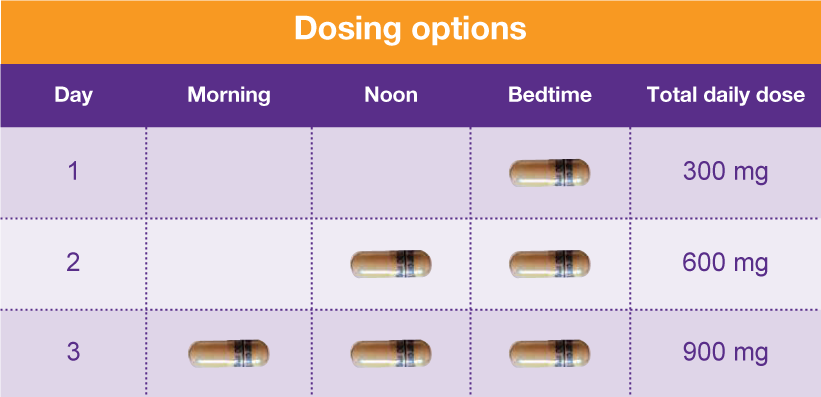
The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Except when dispensed or administered directly by a practitioner or administered by a person authorized to administer by such practitioner, other than a pharmacist, to an ultimate user, no controlled dangerous substance included in Schedule III and IV which is a prescription drug as determined under the Louisiana Revised Statutes may be dispense schedule i A. Opiates. Unless specifically excepted or unless listed in another schedule, any of the following opiates, including their isomers, esters, ethers, salts, or salts of isomers, esters, and ethers, whenever the existence of such isomers, esters, ethers, or salts is possible within the specific chemical designation: The Louisiana State Board of Nursing (LSBN) sent an email on July 17, 2014 to all APRNs licensed in Louisiana who hold prescriptive authority detailing changes in laws regarding Soma and Tramadol. There have also been changes in the classification of hydrocodone products. In the state of Louisiana, Act 397 (SB 618) became effective August 1, 2014. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. gabapentin. The effect of adding these nine drugs to that list will require pharmacies dispensing these drugs to include those dispensing transactions in their automated reports to the state PMP. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. After adjustment, Schedule V gabapentin regulation resulted in a reduction of 8.37 total days of gabapentin prescribed per enrollee (95% confidence interval of - 10.34 to - 6.39). In contrast, PDMP regulation resulted in a reduction of 1.01 total days of gabapentin prescribed per enrollee (95% confidence interval of - 1.74 to - 0.29). This overview explores how Louisiana categorizes Schedule 2 drugs, the criteria used to determine offenses, and the consequences individuals face for violating these laws. Additionally, it addresses possible defenses and exceptions within this legal context. State of Louisiana . January 12, 2022 . M I N U T E S . A meeting of the Louisiana Prescription Monitoring Program (PMP) Advisory Council scheduled to meet on Wednesday, January 12, 2022 at Embassy Suites Hotel, 4914 Constitution Avenue in Baton Rouge, LA 70808, convened at 1:10 p.m. to consider the following: A G E N D A . 1. Call to Order . 2. The Louisiana State Board of Nursing (LSBN) sent an email on July 17, 2014 to all APRNs licensed in Louisiana who hold prescriptive authority detailing changes in laws regarding Soma and Tramadol. There have also been changes in the classification of hydrocodone products. In the state of Louisiana, Act 397 (SB 618) became effective August 1, 2014. Schedule I lists the most dangerous drugs, whereas schedule V contains the least dangerous drugs. Examples of drugs within each schedule include: Schedule I: heroin, LSD, marijuana, mescaline, MDMA, and psilocybin. Schedule II: opium, morphine, oxycodone, fentanyl, and methamphetamine. There are established five schedules of controlled substances, to be known as Schedules I, II, III, IV, and V. Such schedules shall initially consist of the substances listed in R.S. 40:964. In determining that a substance is to be added to these schedules, the secretary shall find the following: A. As to Schedule I: A. As to Schedule I: (1) The drug or other substance has a high potential for abuse. (2) The drug or other substance has no currently accepted medical use in treatment in the United States, and (3) There is a lack of accepted safety for use of the drug or other substance under medical supervision. B. As to Schedule II: If you experience any technical difficulties navigating this website, click here to contact the webmaster. P.O. Box 94062 (900 North Third Street) Baton Rouge, Louisiana 70804-9062 because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin Ohio 12/1/2016 PDMP Reporting for Gabapentin Tennessee 7/1/2018 Schedule V Classification of Gabapentin Virginia 2/23/2017 PDMP Reporting for Gabapentin West Virginia 6/7/2018 Schedule V Classification of Gabapentin Wyoming 7/1/2017 PDMP Reporting for Gabapentin JGIM Grauer and Cramer: Association of State-Imposed Restrictions on Gabapentin 3631 RS 40:964 - Composition of schedules. Schedules I, II, III, IV, and V shall, unless and until added pursuant to R.S. 40:962, consist of the following drugs or other substances, by whatever official name, common or usual name, chemical name, or brand name designated:
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |