Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
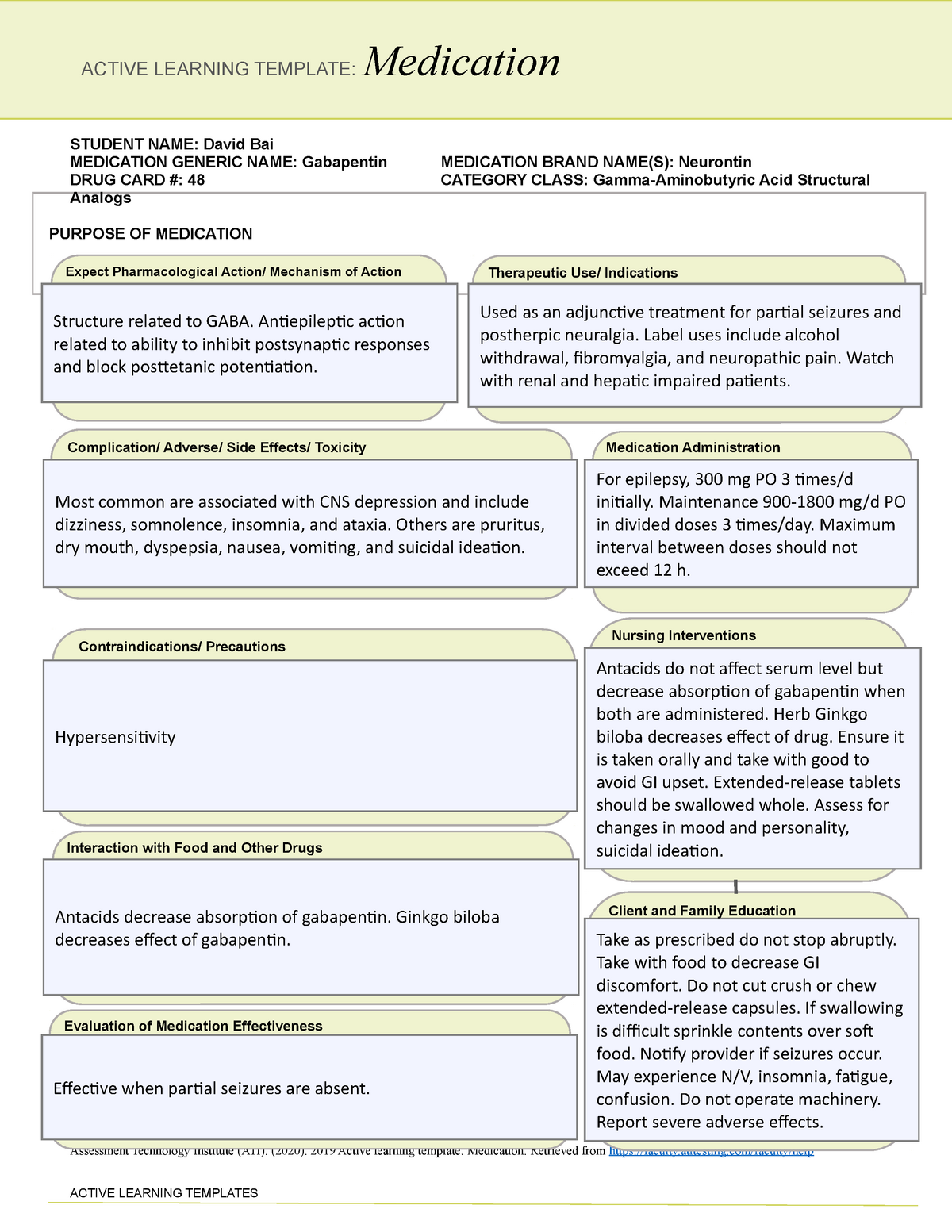
Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. 3333 - Dispensing upon official New York state prescription . 3334 – Emergency oral prescriptions for schedule II drugs and certain other controlled substances 3335 - Dispensing by online dispensers of controlled substances . 3337 - Oral prescriptions schedule Ill, I V, V substances 3338 - Official New York state prescription forms Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid 80.69 Schedule III, IV and V substances 66 80.70 Oral prescriptions for schedule III, IV and V substances 69 80.71 Practitioners; dispensing controlled substances 71 80.72 Issuance of official New York State prescription forms 73 There are hereby established five schedules of controlled substances, to be known as schedules I, II, III, IV and V respectively. Such schedules shall consist of the following substances by whatever name or chemical designation known: Schedule I. The dispensing to a nonhuman patient of gabapentin. New Jersey (NJ) Prescriber actions 30: A pharmacy filling a prescription for gabapentin in an outpatient setting, shall collect and electronically transmit to the Division’s PMP vendor on a daily basis information for each prescription. Exemptions: Subdivision 2 of section 3371 of the public health law is amended by adding a new paragraph (d) to read as follows: (D) FOR PURPOSES OF THIS SECTION, GABAPENTIN (NEURONTIN, GRALISE, HORIZANT, GABARONE) AND ITS CHEMICAL EQUIVALENTS SHALL BE DEEMED TO BE A CONTROLLED SUBSTANCE. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid This bill reclassifies the drug gabapentin as a controlled substance in New York. It amends the public health law to add gabapentin to Schedule VI, which will require it to be monitored through the state's prescription monitoring program. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. SUMMARY OF PROVISIONS: Section one adds a new schedule VI to the public health law that includes non-narcotic drugs such as gabapentin and its equivalents. Sections two and four allow gabapentin and its equivalents to be tracked on the prescription monitoring program. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances having a stimulant effect on the central nervous system, including its salts, isomers (whether optical, position, or geometric), and salts of such isomers whenever the In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Establishes a schedule VI of drugs and other substances; includes gabapentin as a drug to be monitored through the prescription monitoring program; makes conforming changes; and directs the commissioner of health to promulgate regulations necessary or desirable to classify gabapentin and its chemical equivalents as a scheduled substance for the (a) When a patient seeks treatment for any neuromusculoskeletal condition that causes pain, where a practitioner considers an opioid treatment, the practitioner shall consider, discuss with the patient, and, as appropriate, refer or prescribe non-opioid treatment alternatives, based on the practitioner’s clinical judgment and following generally Ioflupane Removed From Controlled Substance Schedules . Effective August 18, 2016, Ioflupane, an injectable radiopharmaceutical diagnostic tool, that is derived from cocoa leaves, and is used in testing for adult patients with suspected Parkinsonism syndromes, was removed from Schedule II of the New York State Controlled Substance Schedules. § 3306. Schedules of controlled substances. There are hereby established five schedules of controlled substances, to be known as schedules I, II, III, IV and V respectively. Such schedules shall consist of the following substances by whatever name or chemical designation known: Schedule I. (a) Schedule I shall consist of the drugs and other Mary Peart, 67, a retired nurse in Manchester-by-the-Sea, Mass., began taking gabapentin a year and a half ago to reduce the pain and fatigue of fibromyalgia. Gabapentin is a popular prescription drug that is approved by the U.S. Food and Drug Administration (FDA) for nerve pain and epilepsy treat- ment. However, through its mechanism of action, the drug boosts the effects of opioids to increase the euphoric effect.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |