Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
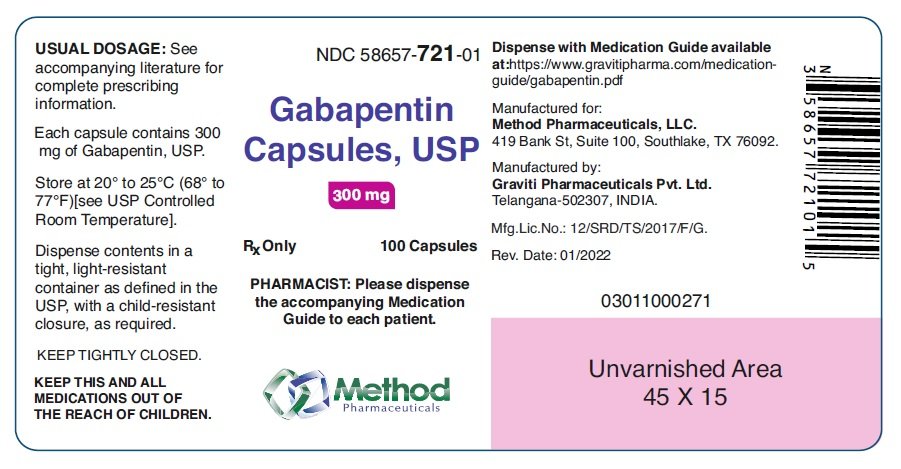
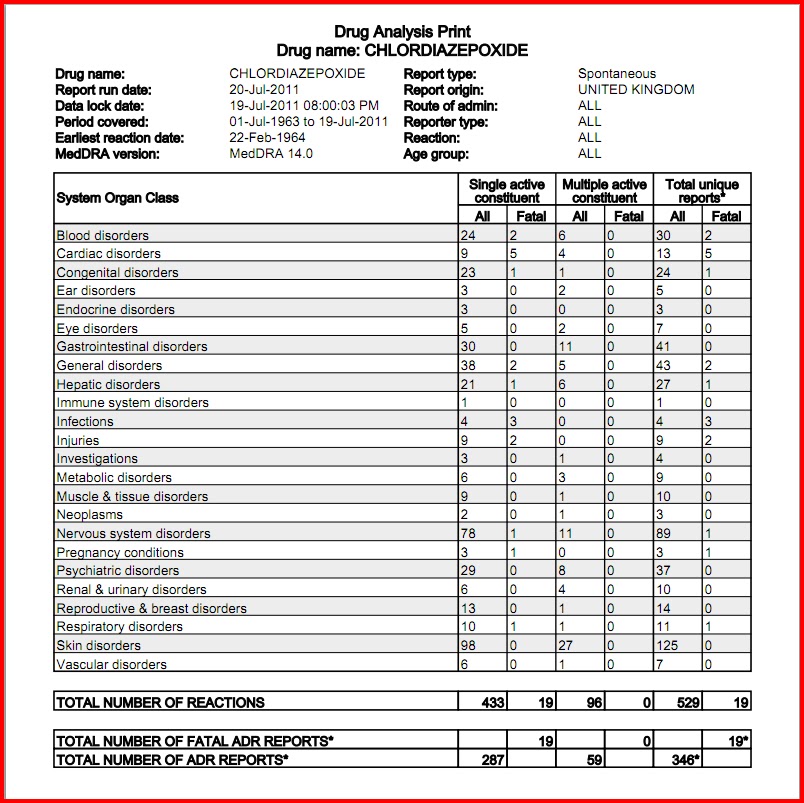
Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation Gabapentin is a prescription medication originally approved to treat seizure and nerve pain disorders. Since its approval, gabapentin has become the drug of choice for many disorders outside of its labeled indication. Gabapentin is generally seen to be a helpful medication with few risks, but it’s still possible for abuse to occur. containing gabapentin to the Ohio Automated Rx Reporting System (OARRS): All pharmacies located outside this state and licensed as a terminal distributor of dangerous drugs that dispense gabapentin to outpatients residing in this state. 77 South High Street, 17th Floor, Columbus, Ohio 43215 T: (614) 466.4143 | F: (614) 752.4836 | contact@pharmacy.ohio.gov | www. pharmacy.ohio.gov. Controlled Substance Reference Table Annual Review Completed for all Drug Entries on 9-15-2019 . Please be advised that the information contained in this table is compiled solely Ohio Board of Nursing | 8995 East Main Street, Reynoldsburg, OH 43068 | Phone: 614-466-3947 or Fax: 614-466-0388 Any pharmacy that is licensed by the Ohio Board of Pharmacy, even if located outside of Ohio, is required to report the dispensing of all Schedule II through Schedule V controlled substance medications, any gabapentin-containing product, and naltrexone when dispensed for treatment of substance abuse disorder to the OARRS database on a daily basis. Established in 2006, OARRS collects information on all outpatient prescriptions for controlled substances and two non-controlled substances (gabapentin & naltrexone) dispensed by Ohio-licensed pharmacies and personally furnished by Ohio prescribers. This data is reported every 24 hours and is maintained in a secure database. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. schedule II controlled substances. IMPORTANT INFORMATION ON REFILLS: Prescriptions for schedule III and IV controlled substances may be refilled not more than five times in a six-month period from issuance for schedule V controlled substances and for dangerous drugs that are not controlled substances. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- registration numbers for gabapentin prescriptions issued by veterinarians. As a reminder, gabapentin is not a controlled substance in Ohio and so a veterinarian is not required to have a DEA registration number to prescribe the medication. Additionally, veterinarians do not have NPI numbers. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. %PDF-1.4 %âãÏÓ 43 0 obj > endobj xref 43 90 0000000016 00000 n 0000002450 00000 n 0000002561 00000 n 0000003887 00000 n 0000004026 00000 n 0000004206 00000 n 0000004714 00000 n 0000005176 00000 n 0000005672 00000 n 0000005785 00000 n 0000005810 00000 n 0000006414 00000 n 0000006688 00000 n 0000007218 00000 n 0000007492 00000 n 0000007985 00000 n 0000010362 00000 n 0000010605 00000 n Gabapentin, a drug viewed as an alternative to opioids, is being abused across Ohio, experts and state officials warn. The misuse could lead the state to reclassify the drug. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Required OARRS Review for Gabapentin Prescriptions - Effective 11/15/22 Effective November 15, 2022, OAC 4729:5-5-08 will be amended to require pharmacists to check a patient’s OARRS report prior to dispensing medications containing gabapentin. This rule is intended to address an increase in the overutilization of gabapentin as reported by Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. State Medical Board of Ohio | 30 East Broad Street, 3rd Floor, Columbus, OH 43215 | Call: 614-466-3934 Controlled Substance Reference Table . Annual Review Completed for all Drug Entries on 9-15-2022 . Please be advised that the information contained in this table is compiled solely from reference works recognized and approved by the State Board of Pharmacy pursuant to rule 4729:9-2-01. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |