Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
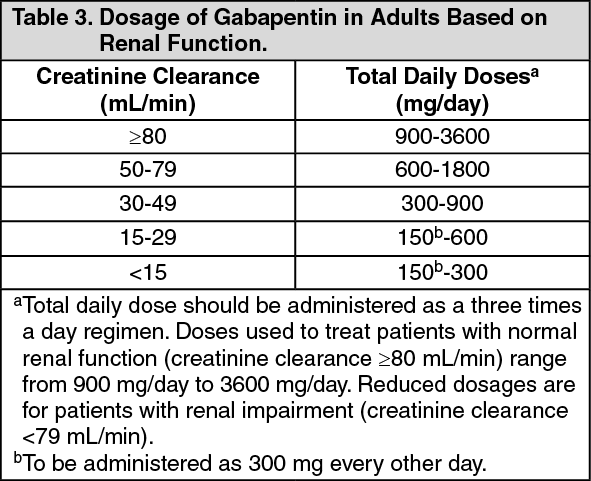
Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Oregon Administrative Rules Division 80, Schedule of Controlled Substances. Refreshed: 2021-06-08 Prescription Drug Monitoring Program PO Box 14450 Portland, OR 97293-0450 Phone: 971-673-0741 TTY: 971-673-0372 E-mail: pdmp.health@odhsoha.oregon.gov Patients are encouraged to talk with their healthcare providers regarding their prescription medications. Oregon 23: 01/01/2020: Gabapentin reported under Oregon PDMP: HB 2257: Utah 24: 11/01/2019: Gabapentin tracked by Utah Controlled Substance Database: HB 449 Rule R156-37f-203(8) Wyoming 25: 07/2017: Gabapentin reporting to WORx: W.S. 35-7-1001-1101 Future deliberations Delaware 26: Deliberations to classify gabapentin as a Schedule V controlled Pharmacies submit prescription data to the PDMP system for all Schedules II, III and IV controlled substances, gabapentin, and naloxone dispensed from Oregon pharmacies and to Oregon residents from non-resident pharmacies. The protected health information is collected and stored securely. Oregon Laws & Rules The following is a list of resources to provide the most up-to-date information on laws and rules governing the practice of pharmacy and controlled substances: Oregon Revised Statutes - Chapter 689; Oregon Revised Statutes - Chapter 475; Oregon Administrative Rules - Chapter 855; Recent Rule changes; Drug Enforcement Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Constraints Still Remain in Oregon’s Prescription Drug Monitoring Program • US Food and Drug Administration o FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) • Other Articles o How Gabapentin Differs From Pregabalin. Gabapentin is not scheduled as a controlled substance, meaning that the medication has little potential for addiction and abuse. However, the drug does show characteristics of various medications associated with misuse and addiction such as benzodiazepines, producing similar withdrawal syndromes and psychoactive effects. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Prescribers and pharmacists are only allowed to search information regarding patients under your care. The PDMP collects data on Federally Scheduled II, III, and IV controlled substances, State Scheduled drugs including pseudoephedrine, state drugs of interest gabapentin and naloxone. 855-080-0022 Schedule II Schedule II consists of the drugs and other substances by whatever official, common, usual, chemical, or brand name designated, listed in 21 CFR 1308.12 (04/01/2023) and any quantity of methamphetamine, when in the form of a FDA approved product containing methamphetamine, its salts, isomers, and salts of its isomers as an active ingredient for the purposes of As a result, gabapentin doses greater than 1,800 mg/day don’t appear to provide additional neuropathic pain relief. Gabapentin may help attenuate withdrawal symptoms from alcohol or opioids, and abusers will often “bridge” with gabapentin until they can obtain a supply of illicit drugs. The Oregon PDMP collects data on Schedules II, III and IV controlled substances and gabapentin. Beginning Jan 2025, the PDMP will begin collecting schedule V drugs and veterinarian prescribed controlled substances. Any person who violates this subsection with respect to: A controlled substance in Schedule I, is guilty of a Class A felony, except as otherwise provided in ORS 475.886 (Unlawful manufacture of methamphetamine) and 475.890 (Unlawful delivery of methamphetamine). APRNs shall only prescribe the controlled substances from Schedules II–V, at the level provided for on their DEA certificate. Nurse Practitioners who treat opioid addiction must demonstrate that they meet federal requirements and obtain a waiver from the Substance Abuse and Mental Health Services Administration (SAMHSA). Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. this is a joint statement from the oregon medical board and the oregon board of pharmacy regarding electronic prescriptions for controlled substances. The mission of the Oregon Medical Board is to protect the health, safety, and wellbeing of Oregon citizens by regulating the practice of medicine in a manner that promotes access to quality care. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |