Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 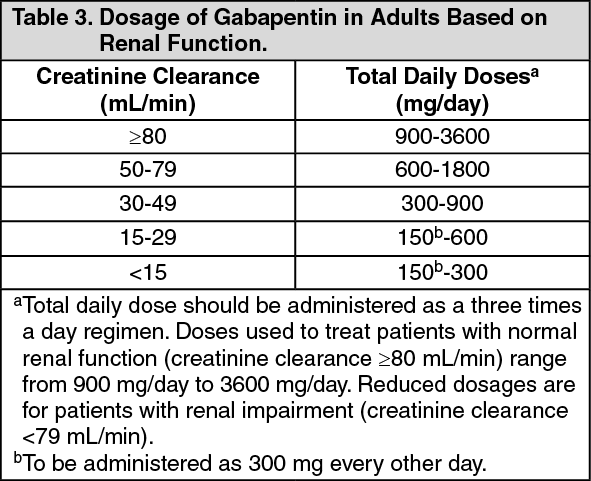 |
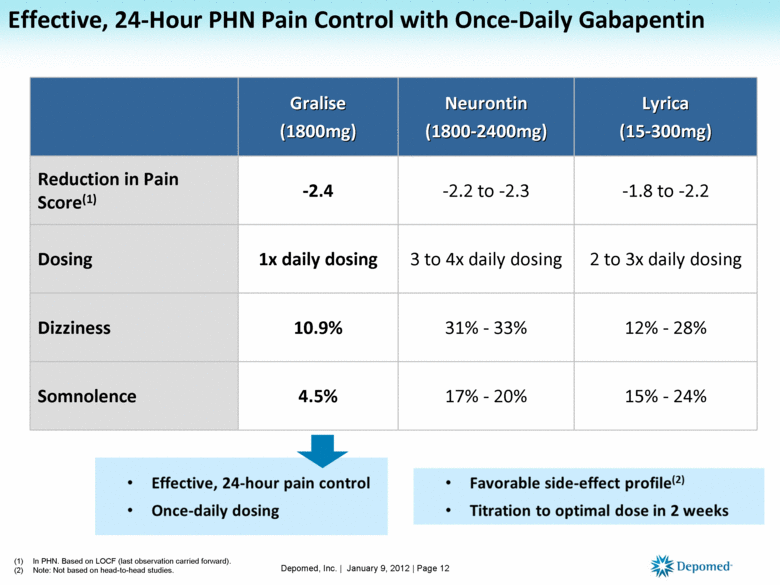
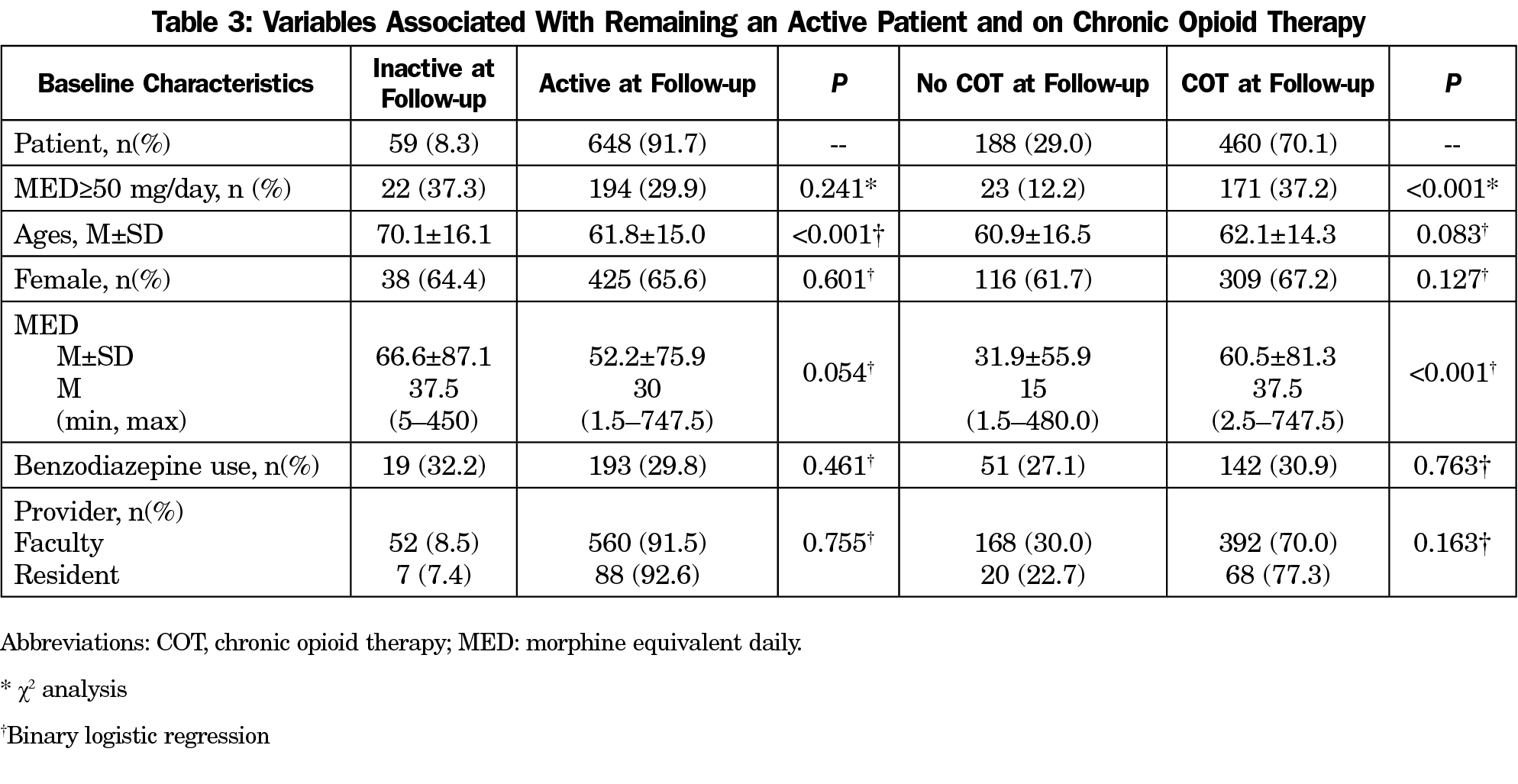
ective May 1, 2024, all Gabapentin products will be Schedule V controlled substances in Utah. All applicable provisions of recently passed Utah House Bill 260 CONTROLLED SUBSTANCES AMENDMENTS that a ected UCA 58-37-4 Schedules of controlled substances and other licensure board regulations will apply to Gabapentin. Yes, Gabapentin is a Schedule V Controlled Substance in Tennessee and should be reported to the CSMD. NOTE: The above frequently asked questions and answers do not supersede the terms of the law governing the CSMD, but are merely provided as guidance for purposes of implementation and enforcement. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. [It’s] because gabapentin originally was approved for partial onset seizures back in 1993. Following that, a very similar drug, small molecule drug pregabalin, brand name Lyrica, was approved for some similar indications in 2005. Pregabalin was scheduled and is still on schedule V. Gabapentin is not, going back to 1993. So why now? § 90‑93. Schedule V controlled substances. (a) This schedule includes the controlled substances listed or to be listed by whatever official name, common or usual name, chemical name, or trade name designated. In determining that a substance comes within this schedule, the Commission shall find: a low potential for abuse Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. (a) Schedule V consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. (b) Narcotic drugs containing non-narcotic active medicinal ingredients. Any compound, mixture, or preparation containing any of the following narcotic drugs, or their salts States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways For schedules, the rule changes adopt the federal schedule subject to drugs scheduled by the state after January 6, 2022, and the rules promulgated by the Michigan Board of Pharmacy; remove Brorphine, Gabapentin, and Pentazocine as exceptions to the federal schedule; provide an exception to the federal scheduling for isomers, Salvia Divorum Order placing lasmiditan into schedule V. Effective Jun 26, 2020. Published in the June 5, 2020 edition of the Texas Register (45 TexReg 3905) Order placing cenobamate into schedule V. Effective Jun 26, 2020 Published in the June 5, 2020 edition of the Texas Register (45 TexReg 3905) 2020 Schedules of Controlled Substances In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Regulatory authorities are acknowledging gaba-pentin’s abuse potential through actions to reclassify gabapentin as a Schedule V controlled substance and implementing prescription monitoring programs. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Effective July 1, 2017, all gabapentin products will be Schedule 5 controlled substances in Kentucky. All applicable provisions of KRS Chapter 218A, 902 KAR Chapter 55 and other licensure board regulations will apply to gabapentin. Our findings indicate that Schedule V changes to gabapentin implemented in three states significantly reduced gabapentin prescribing behavior in Medicare Part D enrollee prescribers. In contrast, we found a modest decrease in gabapentin prescribing for states that implemented PDMP regulation. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation that contains any quantity of the following substances having a depressant effect on the central nervous system, including its salts: Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. §60A-2-212. Schedule V. (a) Schedule V shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |