Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
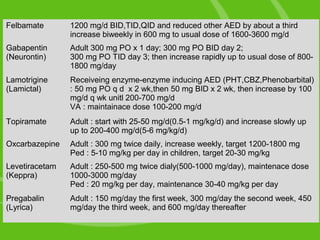 |  |
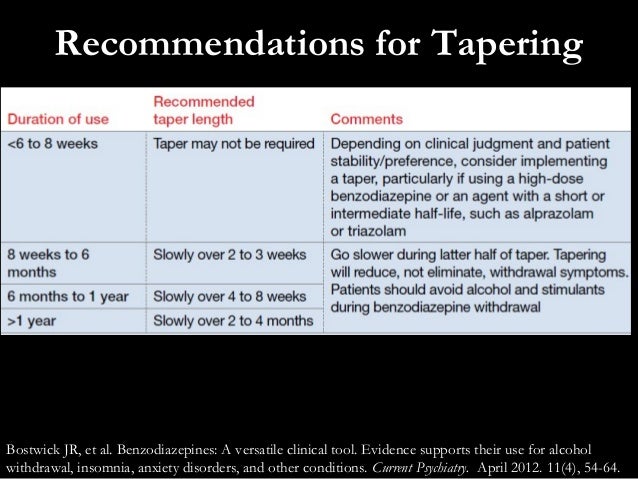
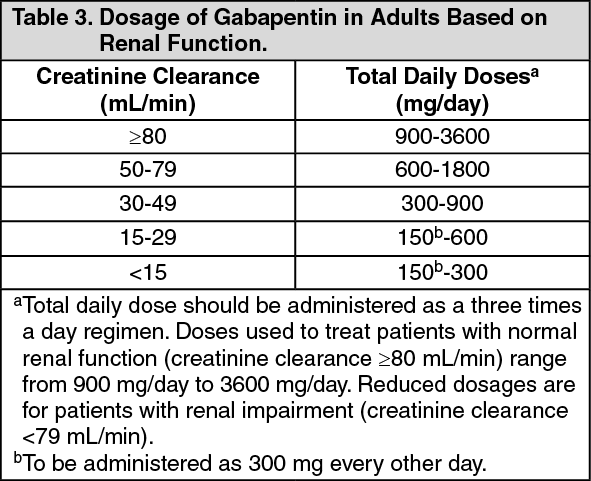
Virginia (VA) Reporting 44: Gabapentin must be reported within 24 hours or the dispenser’s next business day. The program also requires non-resident pharmacies to report dispensing of gabapentin to Virginia residents. Exemptions 45: Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The 2019 Virginia General Assembly recently passed a new bill that will classify gabapentin as a Schedule V substance starting July 1, 2019. Currently, gabapentin is classified as a Schedule VI drug of concern. This change in schedule will require gabapentin to be reported to the Virginia Prescription Monitoring Program as a schedule V substance. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. A BILL to amend and reenact §§ 54.1-3454 and 54.1-3456.1 of the Code of Virginia, relating to Drug Control Act; Schedule V; gabapentin. Be it enacted by the General Assembly of Virginia: 1. That §§ 54.1-3454 and 54.1-3456.1 of the Code of Virginia are amended and reenacted as follows: § 54.1-3454. Schedule V. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances having a stimulant effect on the central nervous system, including its salts, isomers and salts of isomers: In Virginia, gabapentin is classified as a Schedule V controlled substance under section 54.1-3454. This classification places it among substances with a lower potential for abuse relative to those in Schedules I through IV. The 2019 Virginia General Assembly passed HB2557 which classifies gabapentin as a Schedule V controlled substance as of July 1, 2019. Until then, gabapentin remains a drug of concern classified as a Schedule VI controlled substance. The following classes of drugs and devices shall be controlled by Schedule VI: 1. Any compound, mixture, or preparation containing any stimulant or depressant drug exempted from Schedules III, IV or V and designated by the Board as subject to this section. Gabapentin grew to 64 million prescriptions in the United States in 2016 from 39 million prescriptions in 2012, 12 making it the 10th most prescribed medication that year. 13 Gabapentin has been widely recognized as a drug related to opioid abuse in West Virginia. 14 Studies have shown that gabapentin in toxicology reports constituted a Unless specifically excepted or listed in another schedule, any material, compound, mixture, or preparation that contains any quantity of the following substances having a stimulant effect on the central nervous system, including its salts, isomers, and salts of isomers: In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death. 24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Gabapentin, a Schedule V drug in the Commonwealth, had previously been considered a “drug of concern” in Virginia. The 2019 Virginia General Assembly passed HB2557, which classified gabapentin as a Schedule V controlled substance as of July 1, 2019. 13. Are there any special dispensing or prescribing considerations for gabapentin? As of July 1, 2019, gabapentin is a Schedule V controlled substance in Virginia. The Drug Enforcement Administration (DEA) has not yet scheduled gabapentin. Therefore, a §60A-2-212. Schedule V. (a) Schedule V shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Ohio 12/1/2016 PDMP Reporting for Gabapentin Tennessee 7/1/2018 Schedule V Classification of Gabapentin Virginia 2/23/2017 PDMP Reporting for Gabapentin West Virginia 6/7/2018 Schedule V Classification of Gabapentin Wyoming 7/1/2017 PDMP Reporting for Gabapentin JGIM Grauer and Cramer: Association of State-Imposed Restrictions on Gabapentin 3631 WV Board of Pharmacy 1207 Quarrier Street, 4th Floor Charleston, WV 25301 Phone: 304-558-0558 Fax: 304-558-0572 Email: Contact Form | boardofpharmacy@wv.gov Schedule IV. The controlled substances listed in this section are included in Schedule IV unless specifically excepted or listed in another schedule: 1. Any material, compound, mixture, or preparation which contains any quantity of the following substances having a potential for abuse associated with a depressant effect on the central nervous
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |