Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
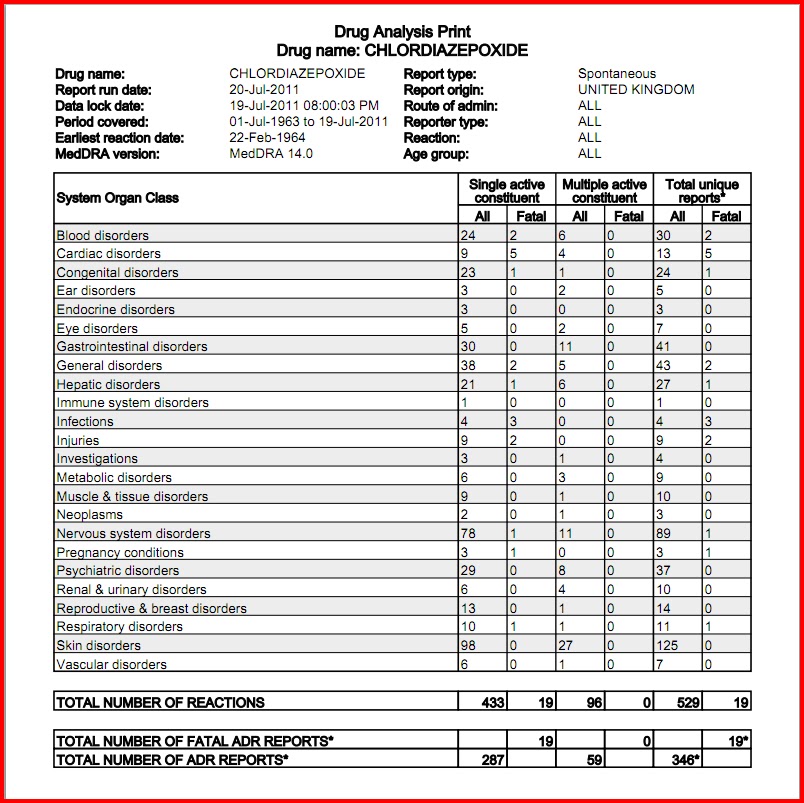
Note that these alerts were not available in the WI PDMP prior to January 17, 2017, but the criteria to meet the alerts were applied to data from previous years to give an indication of how many alerts would have existed. Gabapentin is designated in Wisconsin as a monitored prescription drug effective September 1, 2021. Practical Impact for Many Prescribers and Dispensers of Gabapentin Because Gabapentin has been designated a monitored prescription drug, a practitioner now must review a patient’s prescription drug history as required by Wis. Admin. Code § CSB 4.105 prior to prescribing Gabapentin. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. The Controlled Substances Board reviewed research of the affects of gabapentin. In addition, the Controlled Substances Board took notice of the actions of several s p tates, including our surrounding states, to either schedule gabapentin as a controlled substance or to designate it as a monitored drug in the prescription monitoring programs. As of May 9, 2024, in addition to any controlled substance identified in Schedule II, III, IV and V of the federal controlled substances act the Wisconsin CSB has also identified Gabapentin as having substantial potential for abuse. The patient is receiving hospice care. The prescription is for a three-day or less supply with no refills. As of April 2017, Wisconsin state law requires prescribers to review the PDMP before prescribing any controlled substance for greater than a three-day supply. 6. Prescribing of opioids is strongly discouraged in patients taking benzodiazepines or other respiratory depressants (gabapentin, lyrica, muscle relaxants, sleep aids). 961.18 Schedule III. 961.19 Schedule IV tests. 961.20 Schedule IV. 961.21 Schedule V tests. 961.22 Schedule V. 961.23 Dispensing of schedule V substances. 961.235 Records relating to sales of pseudoephedrine products. 961.24 Publishing of updated schedules. 961.25 Controlled substance analog treated as a schedule I substance. SUBCHAPTER III Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. such as Gabapentin, as a reportable drug. Iowa has not scheduled Gabapenin as a controlled substance. Michigan: Michigan has scheduled Gabapentin as a Schedule V controlled substance. Michigan’s prescription monitoring program requires all Schedule II-V controlled substances to be monitored. In addition to our neighboring state of Michigan, Tennessee and Kentucky have scheduled Gabapentin as a Schedule V controlled substance. In addition to our neighboring state of Minnesota, approximately 8 other states designate Gabapentin as a monitored drug to be reported to prescription drug monitoring programs. 3. Related to: Designating gabapentin as a monitored drug. Date Rule Approved by Governor: March 11, 2021. Date Rule Filed with LRB: July 15, 2021. Scheduled Publication Date: August 30, 2021. Scheduled Effective Date: September 1, 2021 Wisconsin Administrative Code. Controlled Substances Board. Chapter CSB 2 - Additions to Schedules in SS. 961.14, 961.16, 961.18, 961.20 and 961.22, Stats. Chapter CSB 3 - Special Use Authorization Chapter CSB 4 - Prescription Drug Monitoring Program. Wisconsin Statutes. Chapter 15 - Structure of the executive branch Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. 2015 Wisconsin Act 269 granted authority to the Board of Nursing to issue guidelines regarding best practices in prescribing controlled substances, as defined in s. 961.01 (4), Stats., for persons credentialed by the Board of Nursing who are authorized to prescribe controlled substances. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. 961.16(2)(a) (a) Opium and substances derived from opium, and any salt, compound, derivative or preparation of opium or substances derived from opium. Apomorphine, dextrorphan, nalbuphine, butorphanol, naldemedine, nalmefene, naloxegol, naloxone, 6-beta-naltrexol, and naltrexone and their respective salts and the isoquinoline alkaloids of opium and their respective salts are excluded from this Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Always verify the schedule of combination products. Contact pharmacist if there is a question. Schedule III: A drug with medical use and a medium risk of misuse. Example: Certain kinds of steroids; Schedule IV: A drug with medical use and a low risk of misuse. Example: Anxiety medication like Xanax; Schedule V: A drug with medical use and a lower risk of misuse than schedule IV. Example: Over-the-counter cough medication 2013 Wisconsin Act 267, also known as Lydia's Law, charges the Controlled Substances Board with aiding a physician in applying for and processing an investigational drug permit for use of cannabidoil (CBD oil) as treatment for a seizure disorder.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |