Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
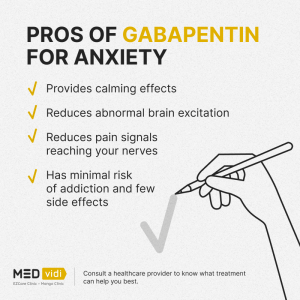
Overall, the risk of being hospitalized with altered mental status after initiating gabapentin remains low, but may be reduced through the judicious use of gabapentin, use of the lowest dose to control pain, and vigilance for early signs of altered mental status. Side effects were more common with gabapentin (6 in 10) than with placebo (5 in 10). Dizziness, sleepiness, water retention, and problems with walking each occurred in about 1 in 10 people who took gabapentin. Serious side effects were uncommon, and not different between gabapentin and placebo. Gabapentin and pregabalin are medicines that are used to treat epilepsy. The neural mechanisms of epilepsy and nerve damage pain have some commonality so the medicines are also prescribed for the treatment of neuropathic (nerve damage) pain such as pain after shingles, diabetes nerve pain and sciatica. They often considered together as ‘gabapentinoids’. High-dose gabapentin is associated with a twofold increase in adverse effects, including somnolence, tremors, ataxia and nystagmus. Note: GABA = γ-aminobutyric acid, OR = odds ratio. The most common side effects of gabapentin are dose related and include dizziness, somnolence, tremor, ataxia, blurred vision, anxiety, and gastrointestinal upset or nausea. Rare, but potentially severe adverse events include hypersensitivity reactions such as angioneurotic edema, drug rash with eosinophilia with systemic manifestations (DRESS Results: The review identified a spectrum of atypical side effects associated with gabapentin use, ranging from neurological manifestations like myoclonus and ataxia to behavioral changes such as pediatric aggression and suicidal ideation. Some of the side effects caused by gabapentin are teratogenicity, hypoventilation, respiratory failure and myopathy. Finally, reports in general contraindicate the use of gabapentin in conditions such as myasthenia gravis and myoclonus. Myoclonus is a rare side effect of gabapentin (GBP) and has been reported in patients with preexisting myoclonus, mental retardation, chronic static encephalopathy, diffuse brain damage, impaired renal function, or end stage renal disease. Side effects associated with gabapentin include somnolence, dizziness, peripheral edema and gait disturbances. Gabapentinoids (including gabapentin) in high doses may result in sedative and psychedelic effects. Gabapentin and lamotrigine are the only two drugs that approved to be more effective than carbamazepine in elderly patients with stroke because they don’t interact with anticoagulants and antiplatelet agents that are prescribed in these patients. 48. Evaluation of serious, uncommon and long-term side effects Gabapentin (GBP) has gained wide acceptance in the treatment of pain, migraine, bipolar illness, and epilepsy. It has a relatively benign side effect profile, lacks significant drug interactions, is not liver metabolized, and is renally excreted. Herein three cases are presented that demonstrate wit In the present case, we discuss the side-effects of gabapentin, used as part of the multimodal treatment approach of painful spinal degenerative disease. The patient stated that he had noticed personality changes after gabapentin was initiated, and that he had become more depressed, frustrated, and aggressive. Gabapentin was initiated at 300 mg/d and titrated to maintenance doses of 1800 to 3600 mg/d by day 12 to 24. The analysis of adverse events was based on 3 distinct groups: patients who received gabapentin <1800 mg/d, those who received gabapentin >or=1800 mg/d, and those who received placebo. Gabapentin can induce diverse side effects such as teratogenicity, hypoventilation, respiratory failure, deficits in visual field, myopathy, self-harm behavior, suicidal behavior, mitochondrial toxicity, somnolence, dizziness and asthenia; these can be related to the route of administration. This activity outlines the indications, mechanisms of action, administration, significant adverse effects, contraindications, monitoring, and characteristics of gabapentin toxicity. There is increasing evidence of the potential to cause harm in vulnerable populations such as the elderly and increasing prevalence of abuse. The risk of respiratory depression in combination with opioids is of particular concern in the context of the current opioid crisis. There were more adverse effects reported with pregabalin (36 effects) than with gabapentin (22 effects). Six pregabalin studies reported euphoria as a side effect, while no studies reported euphoria with gabapentin. This was the only side effect that may correlate with addictive potential. Adverse Effects. Gabapentin may cause certain adverse effects, which are listed below. Severe reactions: The severe adverse reactions include suicidality, depression, Stevens-Johnson syndrome, anaphylaxis, angioedema, erythema multiforme, rhabdomyolysis, and withdrawal seizure or withdrawal symptoms if the drug is discontinued abruptly. One RCT found side effects in 31% of gabapentin group while 10% of patients taking placebo, including confusion, dizziness, nausea, dry mouth, fatigue, headache, blurred vision, and memory loss17. The other RCT reported adverse events in 40% of gabapentin group vs 23.1% in the placebo group, with similar side effects as Ryan et al. 17 reported. When compared with the nondrug condition, both medications resulted in significantly worse performance on tests of verbal memory and motor speed, and participants reported a high incidence of sedative effects. Frequently reported side-effects for gabapentin included fatigue, unsteadiness, and dizziness.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |