Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
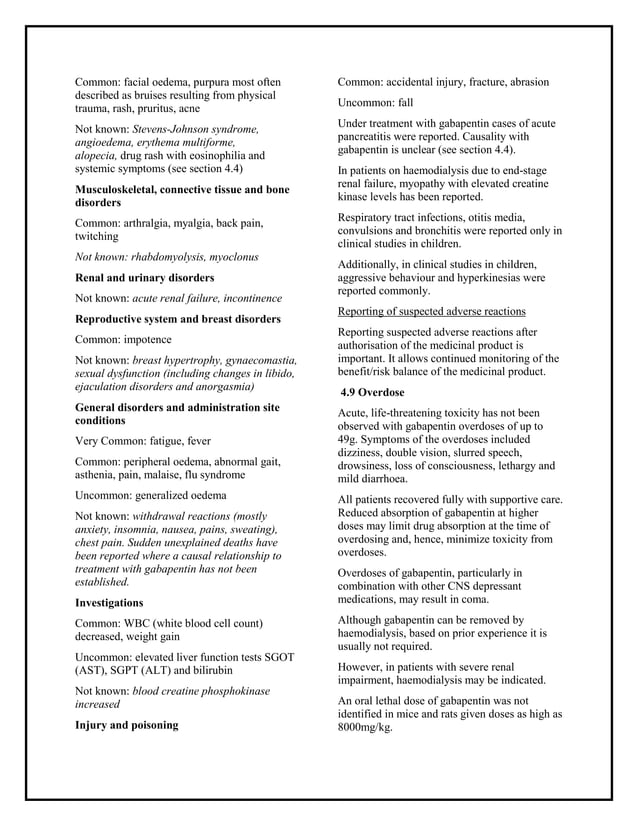
Gabapentin is indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. A comprehensive description of the indications and posology is given in the SmPC. Use gabapentin with cautions as drug reaction with eosinophilla and systemic symptoms (DRESS), a multiogram hypersensitivity with fever, rash or lymphadenopathy has occurred. Gabapentin can cause anaphylaxis and angioedema after first dose or at any time during treatment. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- LYRICA (pregabalin) Capsules, CV LYRICA (pregabalin) Oral Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above (see section 5.1). No dose adjustment is required for patients with hepatic impairment (see section 5.2). Paediatric population . The safety and efficacy of Lyrica in children below the age of 12 years and in adolescents (12-17 years gabapentin should be withdrawn immediately and an alternative treatment considered (as appropriate). If the patient has developed a serious reaction such as SJS, TEN or DRESS with the use of gabapentin, treatment with gabapentin must not be restarted in this patient at any time. Section 4.8 Gabapentin AAA® 400 mg Hartkapseln 2. QUALITATIVE UND QUANTITATIVE ZUSAMMENSETZUNG Jede 100 mg Hartkapsel enthält 100 mg Gabapentin. Jede 300 mg Hartkapsel enthält 300 mg Gabapentin. Jede 400 mg Hartkapsel enthält 400 mg Gabapentin. Sonstiger Bestandteil mit bekannter Wirkung: Jede 100 mg Hartkapsel enthält 22,5 mg Lactose. TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Gabapentin binds with high affinity to the α2δ (alpha-2-delta) subunit of voltage-gated calcium channels and it is proposed that binding to the α2δ subunit may be involved in gabapentin's anti-seizure effects in animals. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a Known hypersensitivity to gabapentin or its ingredients (4) -----WARNINGS AND PRECAUTIONS----- Drug Reaction with Eosinophilia and Systemic Symptoms (Multiorgan hypersensitivity): Discontinue if alternative etiology is not established (5.1) Anaphylaxis and Angioedema: Discontinue and evaluate patient Description of update: Type IB (C.I.2.a) variation application to update SmPC and PIL information in-line with the product information of reference product (Neurontin Hard capsule; EU reference No: DE/H/0899/001; MAH: Upjohn EESV) for Gabapentin Accord 100/300/400 mg hard capsules. PIL sections updated: 4, 6. No changes to SmPC. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly gabapentin dose may be appropriate for individual patients. The minimum time to reach a dose of 1800 mg/day is one week, to reach 2400 mg/day is a total of 2 weeks, and to reach 3600 1. WHAT GABAPENTIN CAPSULES ARE AND WHAT THEY ARE USED FOR Gabapentin capsules, hard (called Gabapentin capsules in the rest of this lea˜et) belong to a group of medicines used to treat epilepsy and peripheral neuropathic pain (long lasting pain caused by damage to the nerves). Gabapentin capsules are used to treat: 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance >60 mL/min) to 52 hours (creatinine clearance <30 mL/min) and Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Fachinformation Gabapentin Glenmark 100 mg Hartkapseln Gabapentin HKP_DE_SmPC_100 mg_V4.0_November 2018 Seite 1 von 10 1. BEZEICHNUNG DES ARZNEIMITTELS Gabapentin Glenmark 100 mg Hartkapseln 2. QUALITATIVE UND QUANTITATIVE ZUSAMMENSETZUNG Jede Hartkapsel enthält 100 mg Gabapentin. Sonstiger Bestandteil mit bekannter Wirkung: Gabapentin is indicated as monotherapy in the treatment of partial seizures with and without secondary generalization in adults and adolescents aged 12 years and above. Treatment of peripheral neuropathic pain
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |