Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
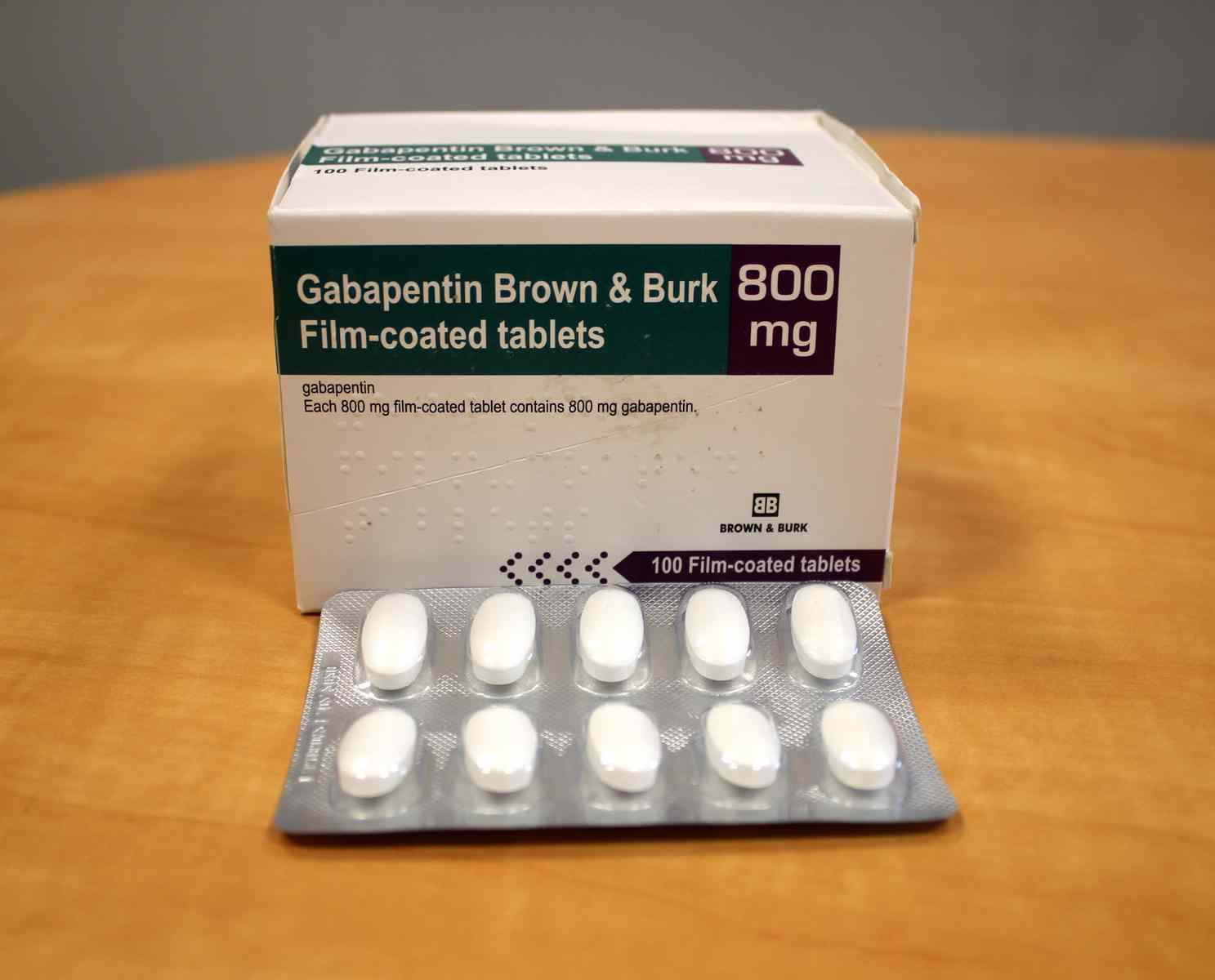 |  |
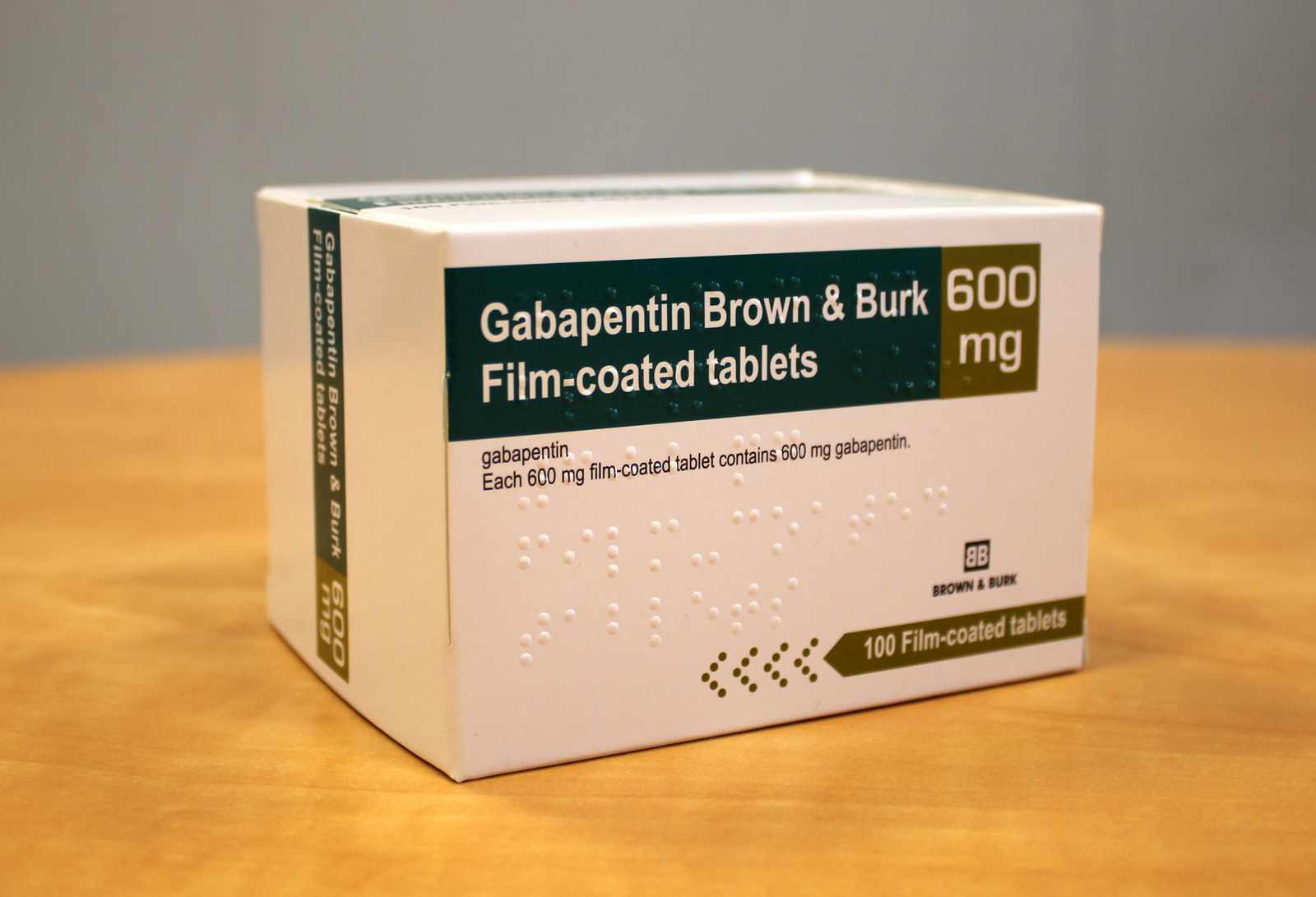
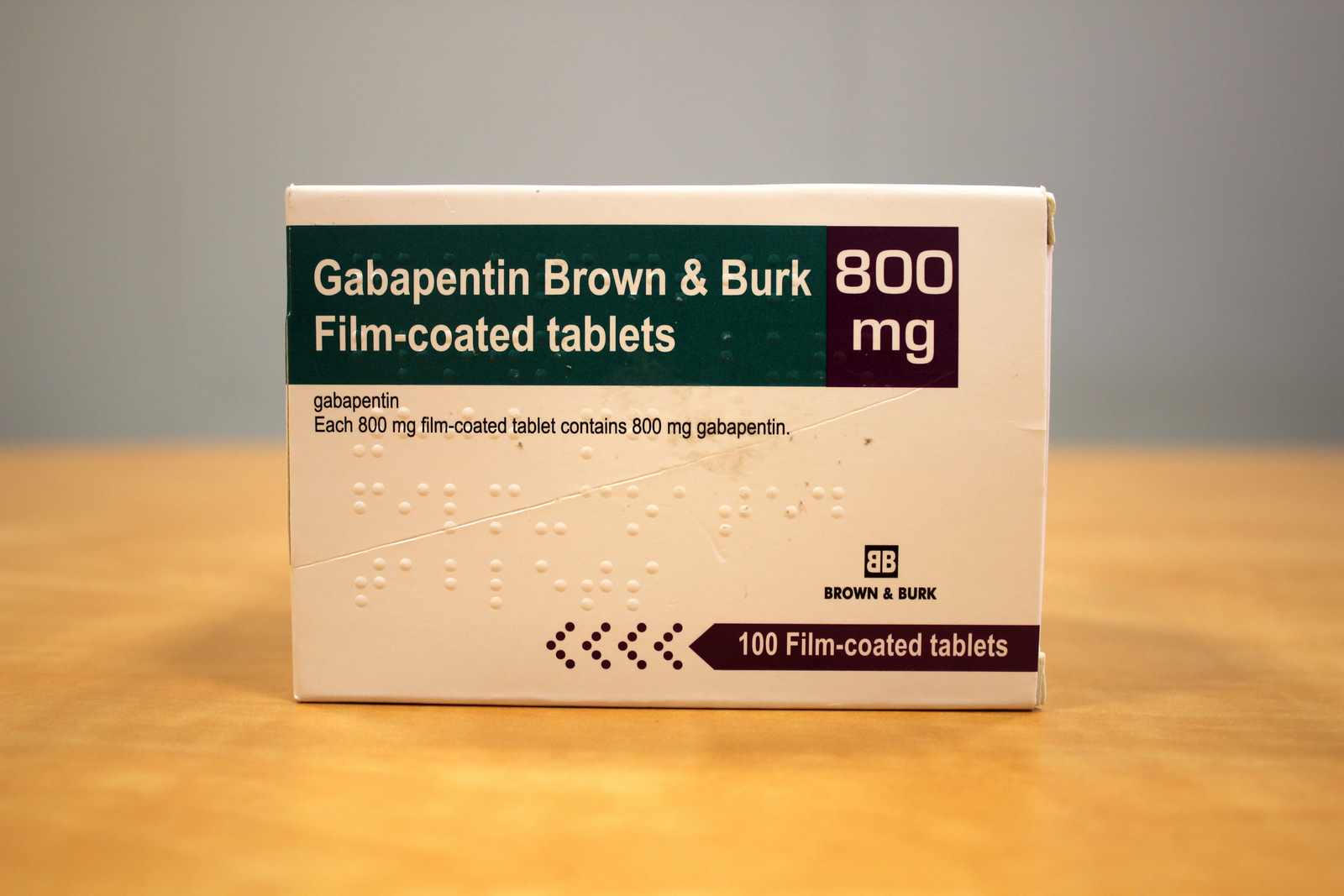
Summary of Product Characteristics for Healthcare Professionals | Upjohn EESV **Product information for a medicine is made up of the Summary of Product Characteristics (SmPC) and Package Leaflet (PL). These documents are issued when a medicine is first licensed for use and are reviewed and updated as necessary throughout the lifetime of a medicine, to reflect the current knowledge about a medicine and risks associated with its use. Gabapentin belongs to a group of medicines used to treat epilepsy and peripheral neuropathic pain (long lasting pain caused by damage to the nerves). The active substance in Gabapentin is gabapentin. Neurontin belongs to a group of medicines used to treat epilepsy and peripheral neuropathic pain (long lasting pain caused by damage to the nerves). The active substance in Neurontin is gabapentin. Gabin belongs to a group of medicines used to treat epilepsy and peripheral neuropathic pain (long lasting pain caused by damage to the nerves). The active substance in Gabin is gabapentin. Various forms of epilepsy (seizures that are initially limited to certain parts of the brain, whether the seizure spreads to other parts of the brain or not). Gabapentin with opioids may cause symptoms like sleepiness and/or decrease in breathing. Gabapentin is not expected to interact with other antiepileptic drugs or the oral contraceptive pill. Gabapentin may interfere with some laboratory tests, if you require a urine test tell your doctor or hospital that you are taking Gabapentin. 1. WHAT GABAPENTIN CAPSULES ARE AND WHAT THEY ARE USED FOR Gabapentin capsules, hard (called Gabapentin capsules in the rest of this lea˜et) belong to a group of medicines used to treat epilepsy and peripheral neuropathic pain (long lasting pain caused by damage to the nerves). Gabapentin capsules are used to treat: Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above (see section 5.1). Gabapentin may have minor or moderate influence on the ability to drive and use machines. Gabapentin acts on the central nervous system and may cause drowsiness, dizziness or other related symptoms. Even, if they were only of mild or moderate degree, these undesirable effects could be potentially dangerous in patients driving or operating Guidance for the veterinary pharmaceutical industry on the production and submission of the Summary of Product Characteristics (SPCs) and product literature mock-ups. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Summary of Product characteristics Health Products Regulatory Authority 15 May 2024 CRN00F68G Page 1 of 12 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Neurostil 100 mg capsules, hard 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Neurostil 100 mg capsules, hard Each capsule contains 100 mg gabapentin For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Gabapentin readily enters the brain and prevents seizures in a number of animal models of epilepsy. Gabapentin does not possess affinity for either GABAA or GABAB receptor nor does it alter the metabolism of GABA. It does not bind to other neurotransmitter receptors of the brain and does not interact with sodium channels. Gabapentin *Additional information is available within the SPC or upon request to the company Scan the QR code or enter an email to access and share this medicine: Gabapentin is present in the breast milk of breast-feeding women. Biotransformation. There is no evidence of gabapentin metabolism in humans. Gabapentin does not induce hepatic mixed function oxidase enzymes responsible for drug metabolism. Elimination. Gabapentin is eliminated unchanged solely by renal excretion. Each 1ml contains 50mg Gabapentin. For a full list of excipients, see section 6.1. The summary of product characteristics (SmPC) is for healthcare professionals. It helps them to prescribe and dispense a medicine. It describes what the medicine is used for, how to take it, possible side effects, who should avoid it, and special precautions to be aware of. Gabapentin (Neurontin and associated names) has been approved in several Member States for the treatment of epileptic syndromes and several types of neuropathic pain. The precise mechanism of action of gabapentin is not known. Gabapentin is structurally related to the neurotransmitter GABA (gammaaminobutyric acid) and interacts with GABA synapses. On 2nd September 2004, Italy (Agencizia
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |