Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
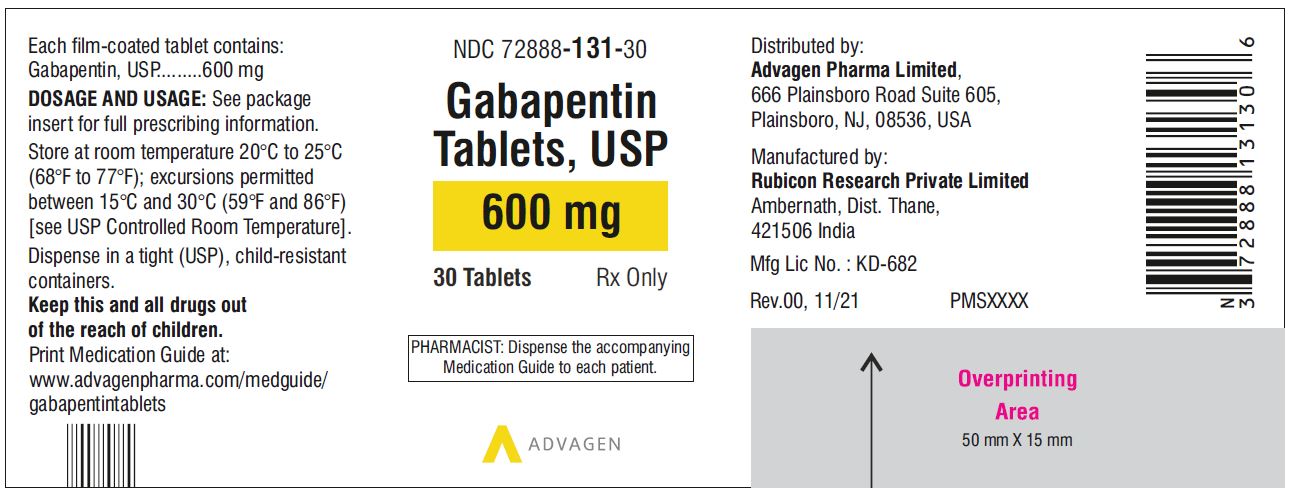
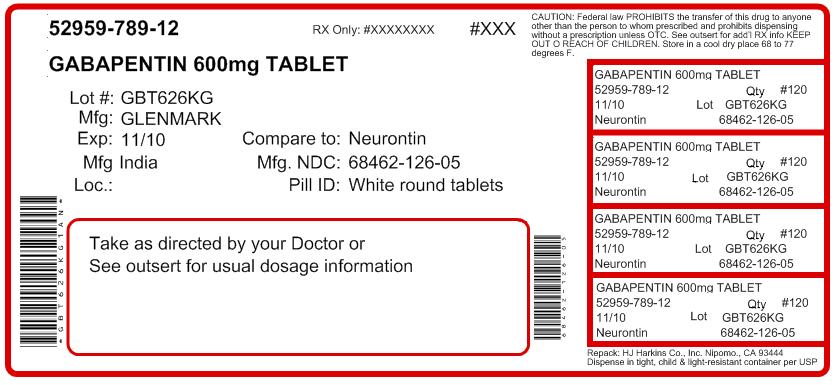
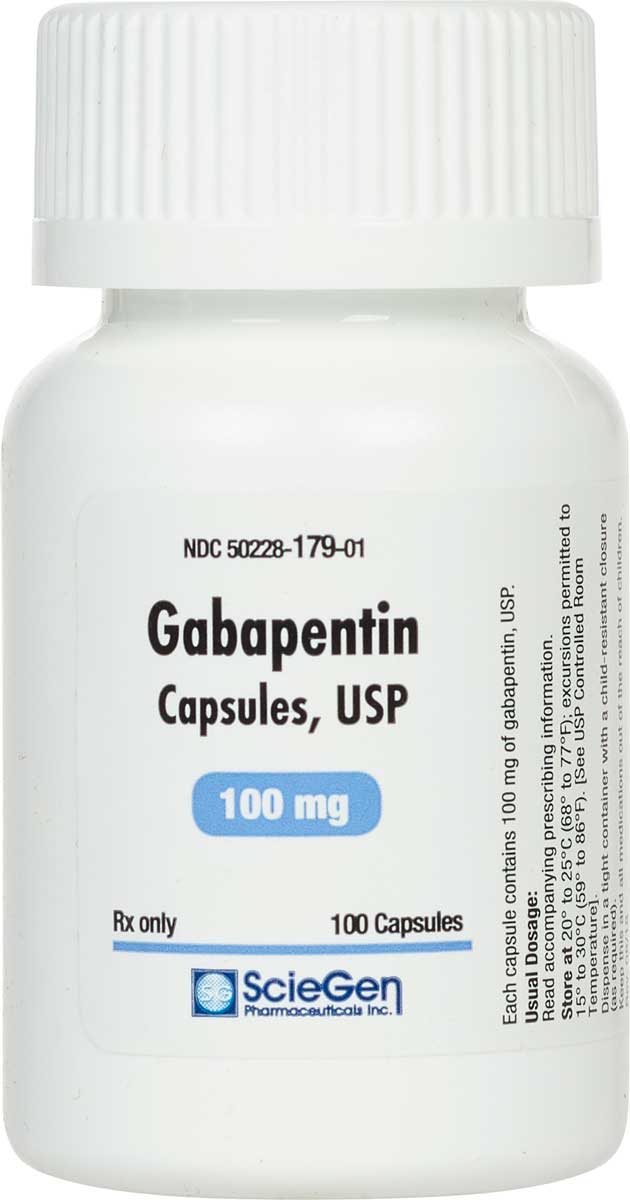
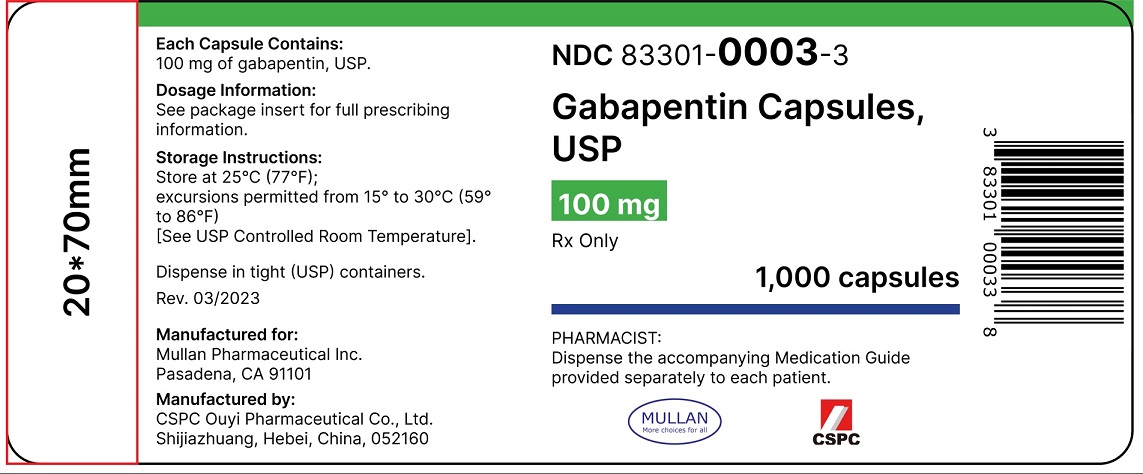
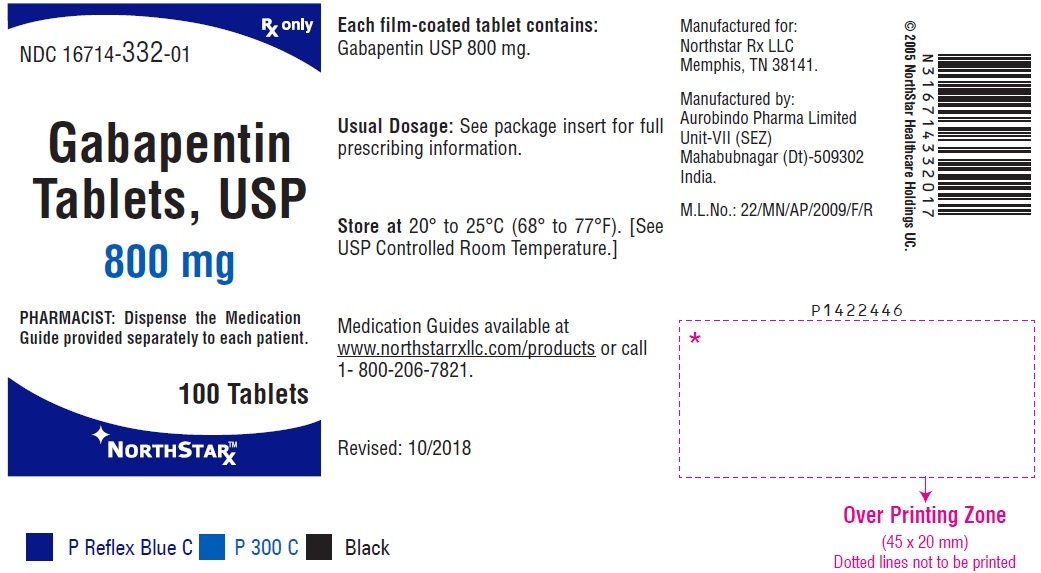
[0003] Gabapentin is 1-(aminomethyl)-1-cyclohexaneacetic acid, having the chemical structure of formula I: 1 [0004] Gabapentin is used for treating cerebral diseases such as epilepsy, faintness attacks, hypokinesis and cranial traumas. The present invention relates to an improved method of preparation of gabapentin, a known pharmaceutical drug, starting from the intermediate 1,1-cyclohexane diacetic acid anhydride via ‘Hofmann’ rearrangement. A pharmaceutical formulation form with improved physical and chemical characteristics, comprising gabapentin in tablet form for oral administration. The tablet form can be prepared by Gabapentin is an anti-epileptic drug indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults with epilepsy. Gabapentin exists Gabapentin can cause a serious condition called multiorgan hypersensitivity or Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome. If experiencing symptoms such as rash, fever, swollen lymph nodes, or liver problems, medical attention should be sought immediately. The present invention provides a pharmaceutical composition of gabapentin wherein lactam level remains below 0.5% even after more than two years of storage at 25 to 30°C and 60% relative humidity. Gabapentin is available as Gralise, Neurontin, and generic gabapentin in the following dosage forms that are taken by mouth. 100 mg, 300 mg, 400 mg oral capsules Some gabapentin tablets should gabapentin tablets containing between 70 and 100% by weight of a granulate as described above and between 0 and 30% by weight, preferably between 0 and 20% of additives Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence gabapentin can be produced in tablet form by Spray-coating gabapentin particles with a binder Solution and compressing the Spray-coated particles into tablets. Gabapentin Abuse. Gabapentin is the drug used for the treatment of epilepsy or seizure disorders. Gabapentin is the generic name of the brand name Neurontin. This drug can be a lot helpful to patient who suffers epileptic disorder. All drugs can be beneficial and therapeutic if given in proper doses and dosages. Considering stopping gabapentin and not sure if it's the right decision? Let us help! Gabapentin is a medication commonly prescribed to treat seizures, nerve pain, and other conditions. Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Camber already offers Gabapentin Tablets and Capsules (generic for Neurontin®). Generics for Gralise® and Neurontin® are not interchangeable. Gabapentin Tablets (generic for Gralise®) are indicated for the management of Postherpetic Neuralgia (PHN). Gabapentin Tablets (generic for Gralise®) 300 mg and 600 mg are available in 90 count bottles. US Patent No. 6,255,526 describes a method of converting gabapentin hydrochloride to gabapentin form II. This patent indicates that prior art methods such as described in US Patent No. Gabapentin oral solution. The oral solution contains 250 millgrams of gabapentin per 5 milliliter (50 mg per mL) Neurontin or generic gabapentin. Gabapentin capsules. It’s available as 100-, 300- or 400-milligram gelatin capsules (Neurontin or generic gabapentin). Gabapentin enacarbil, 300- and 600-milligram extended-release tablets (Horizant). We have now discovered that stable gabapentin tablets can be prepared by a wet granulation method and, unlike the disclosure in U.S. Pat. No. 6,054,482, without having to limit use of gabapentin to a gabapentin having an anion of a mineral acid (calculated as chloride content) less than 20 ppm. The formulations can comprise gabapentin optionally combined with at least one non-opioid pain drug in an aqueous carrier. The pharmaceutical formulation can have a pH of about 2.0 to about 10.0. The at least one non-opioid pain drug can be acetaminophen. Horizant (gabapentin enacarbil) is an extended release tablet used to treat restless legs syndrome and for the pain from having shingles (postherpetic nerve pain). Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older. Warnings The exit time of the tablet from the stomach is related to the absorption of the active substance. As the control of the exit of the products of the patents mentioned above from the stomach by swelling causes alterations in the blood concentrations among the individuals, it is targeted to control the exit from the stomach by more than one method and consequently, in addition to swelling
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |