Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
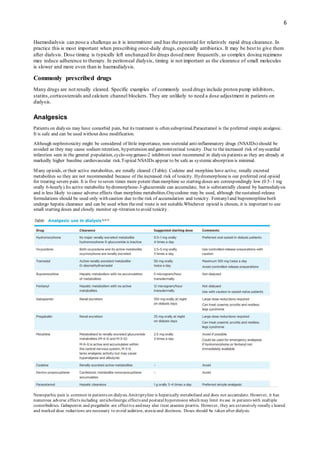
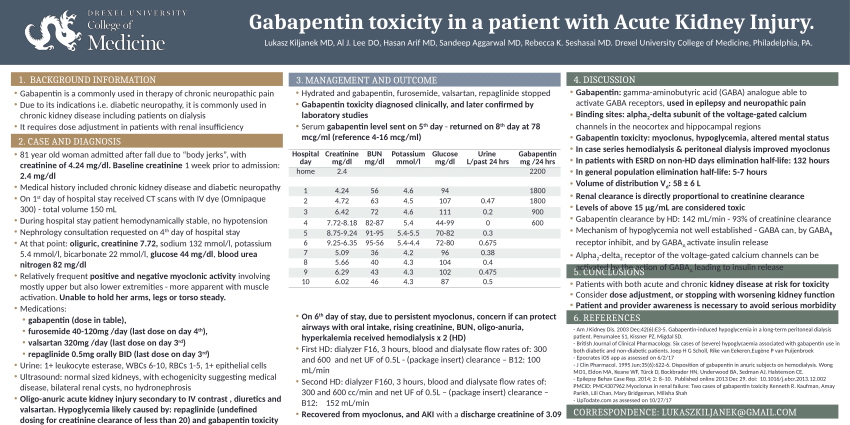
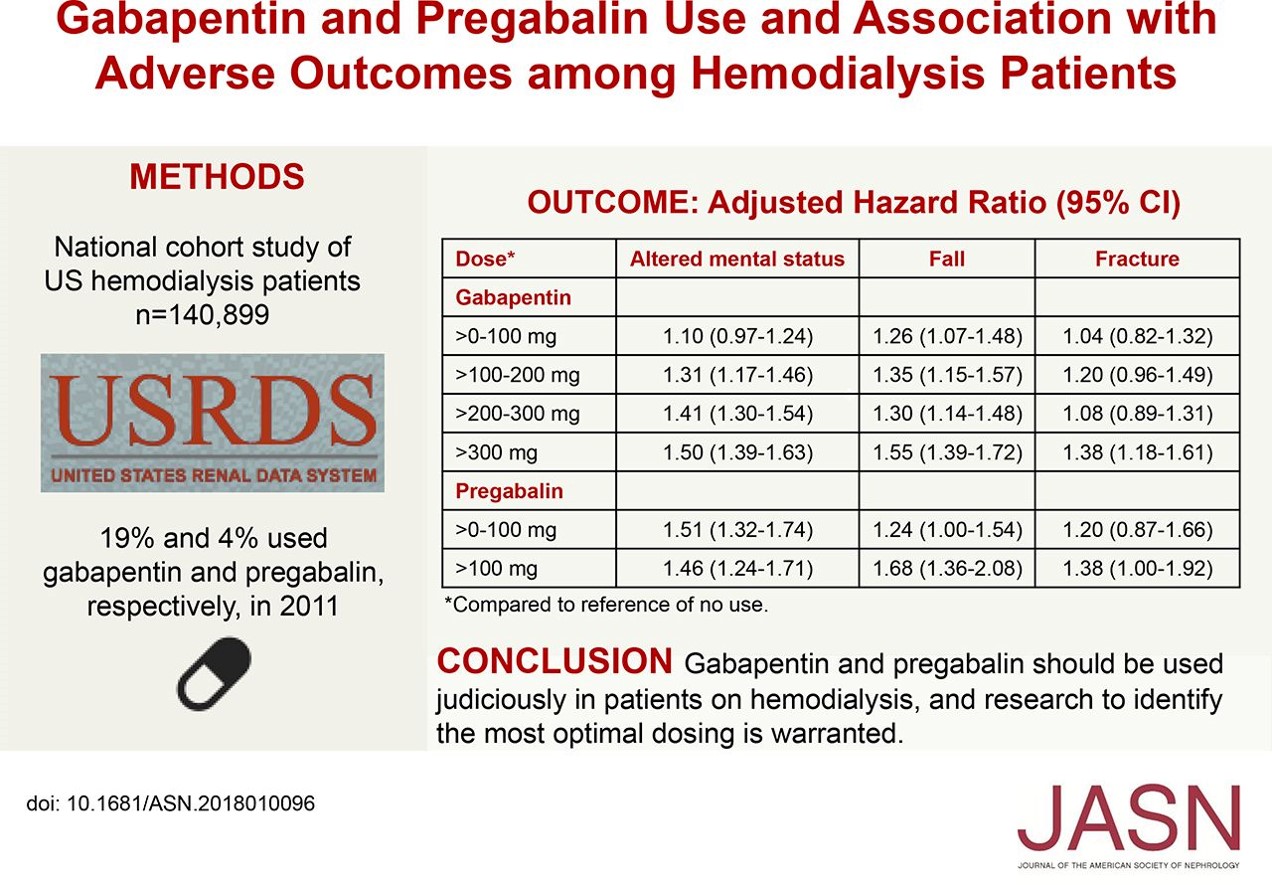
Gabapentin toxicity should be considered one of the differential diagnoses of altered consciousness in patients with compromised renal function, even after a single dose. We report a 57-year-old woman with diabetes mellitus and uraemia on regular Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. Gabapentin is almost exclusively cleared by the kidney and thus presents challenges in patients with kidney failure. Gabapentin is known to be effectively cleared by hemodialysis, but the efficiency of clearance by peritoneal dialysis (PD) has not been previously described. We report a case of gabapentin toxicity in a patient on long-term PD who was treated with continuous automated cycling PD An extreme example is the toxicity among group III patients; all 9 patients receiving dialysis had impressive gabapentin elevation, yet clinical toxicity was evident in 7 patients; the remaining 2 patients with gabapentin levels of 55.0 and 77.2 μg/mL were asymptomatic. We present our experience of 3 cases with Gabapentin toxicity who were managed according to the severity of symptoms. Case 1: A 32-year-old male was found lying unconscious after consuming around Gabapentin is known to be effectively cleared by hemodialysis, but the efficiency of clearance by peritoneal dialysis (PD) has not been previously described. We report a case of gabapentin toxicity in a patient on long-term PD who was treated with continuous automated cycling PD. Renal Dosing; CrCl>60 mL/min: 300-1200mg PO TID CrCl 30-60 mL/min: 200-700mg q12hr CrCl 15-29 mL/min: 200-700mg qDay CrCl<15 mL/min: 100-300mg qDay HD: 125-350mg posthemodialysis after each 4h dialysis interval. Hepatic Dosing Adult: no modifications; Pediatric: no modifications; Contraindications. Allergy to class/drug; Adverse Reactions Serious Introduction. Renal dose adjustments for gabapentin and pregabalin are ubiquitously evident in the medical literature. All manufacturers for these branded and generic dosage forms list dosing recommendations relative to creatinine clearance (CrCl) for both medications (). 1, 2 However, the basis of these recommendations has not been well articulated. Our literature search found only 2 population-based studies examining the association between gabapentin use and risk of toxicity in patients with CKD (our search strategy is shown in Table S2 and the results in Table S3). 11, 14 Only one study examined the risk of toxicity by initial gabapentin dose in new users, 14 and, in a subgroup analysis Discussion: Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. approximately 30% of gabapentin is removed with a 2–4-hour session of he-modialysis (HD) in patients with renal impairment. One study showed a mean 55.6% removal of pregabalin with 4 hours of HD in patients with end-stage renal disease (ESRD) (J lin Pharmacol. 2003 Mar;43(3):277-83). Myoclonic activity may occur as a complication of gabapentin toxicity, especially with acute kidney injury or end-stage renal disease. We report 2 cases of myoclonic activity associated with gabapentin toxicity in the setting of renal disease which resolved with discontinuation of gabapentin and treatment with hemodialysis and peritoneal dialysis. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. Gabapentin is known to be effectively cleared by hemodialysis, but the efficiency of clearance by peritoneal dialysis (PD) has not been previously described. We report a case of gabapentin toxicity in a patient ported with hemodialysis. The workgroup assessed gabapentin and pregabalin as “dialyzable” for patients with decreased kidney function (quality of the evidence grade as A and B, respectively). Limited clinical data were available (24 patients with gabapentin toxicity and 7 with pregabalin toxicity received ECTR). It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Gabapentin is eliminated in urine unmetabolized at a rate proportional to creatinine clearance. 24 In patients with renal impairment, with unaltered gastrointestinal absorption, gabapentin half-life can be prolonged up to 132 hours (without dialysis), 30 placing patients with chronic kidney disease at an increased risk for toxicity. Gabapentin is a relatively safe medication, but in certain clinical scenarios, particularly in impaired renal functions, can lead to severe complications. Moreover, it per se can rarely lead to rhabdomyolysis and AKI. We would like to show you a description here but the site won’t allow us. Myoclonus is a well-reported complication of gabapentin toxicity especially in patients with renal impairment. As gabapentin is solely excreted by the kidneys, renal dose adjustment is recommended in the literature.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |