Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 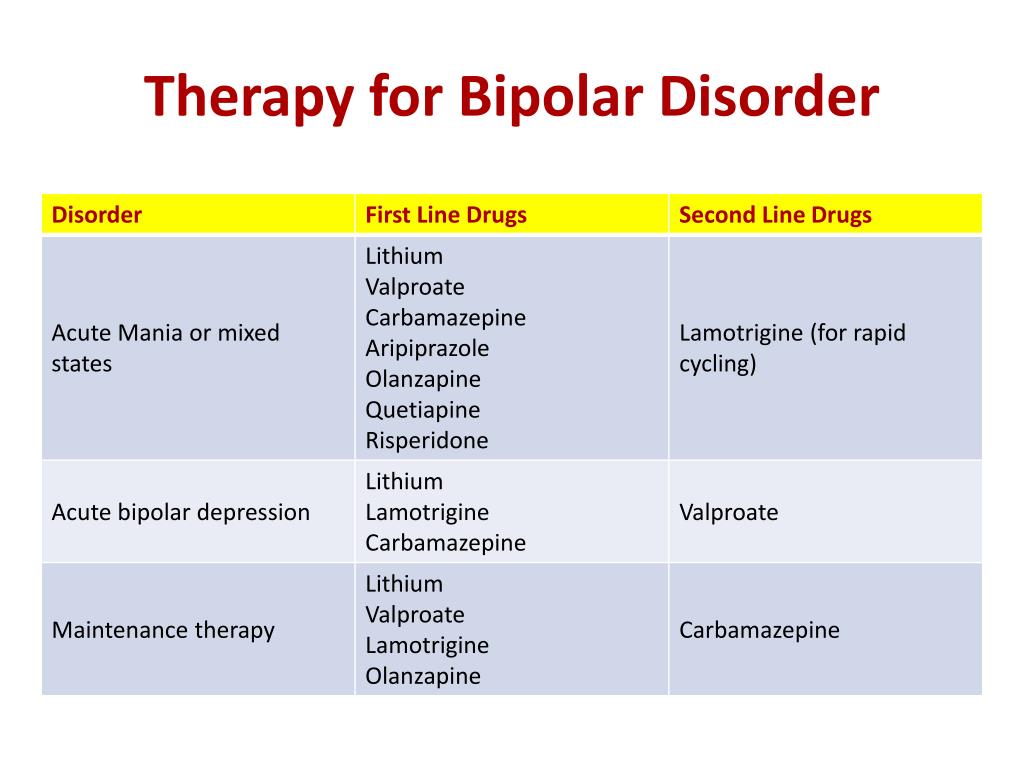 |
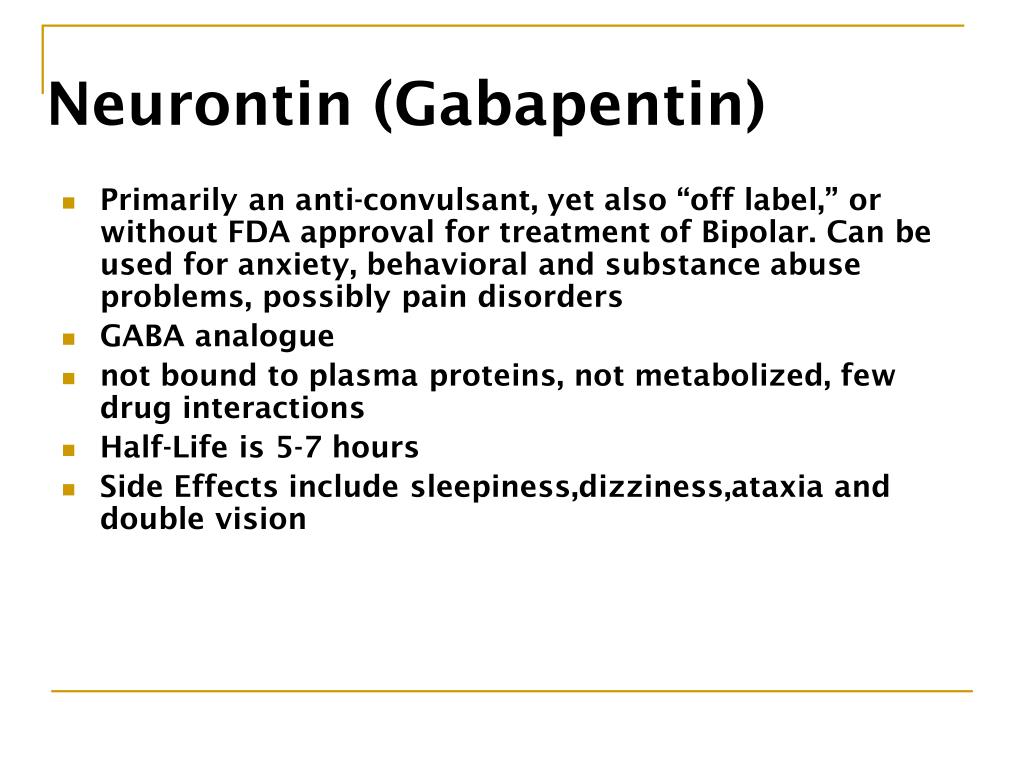
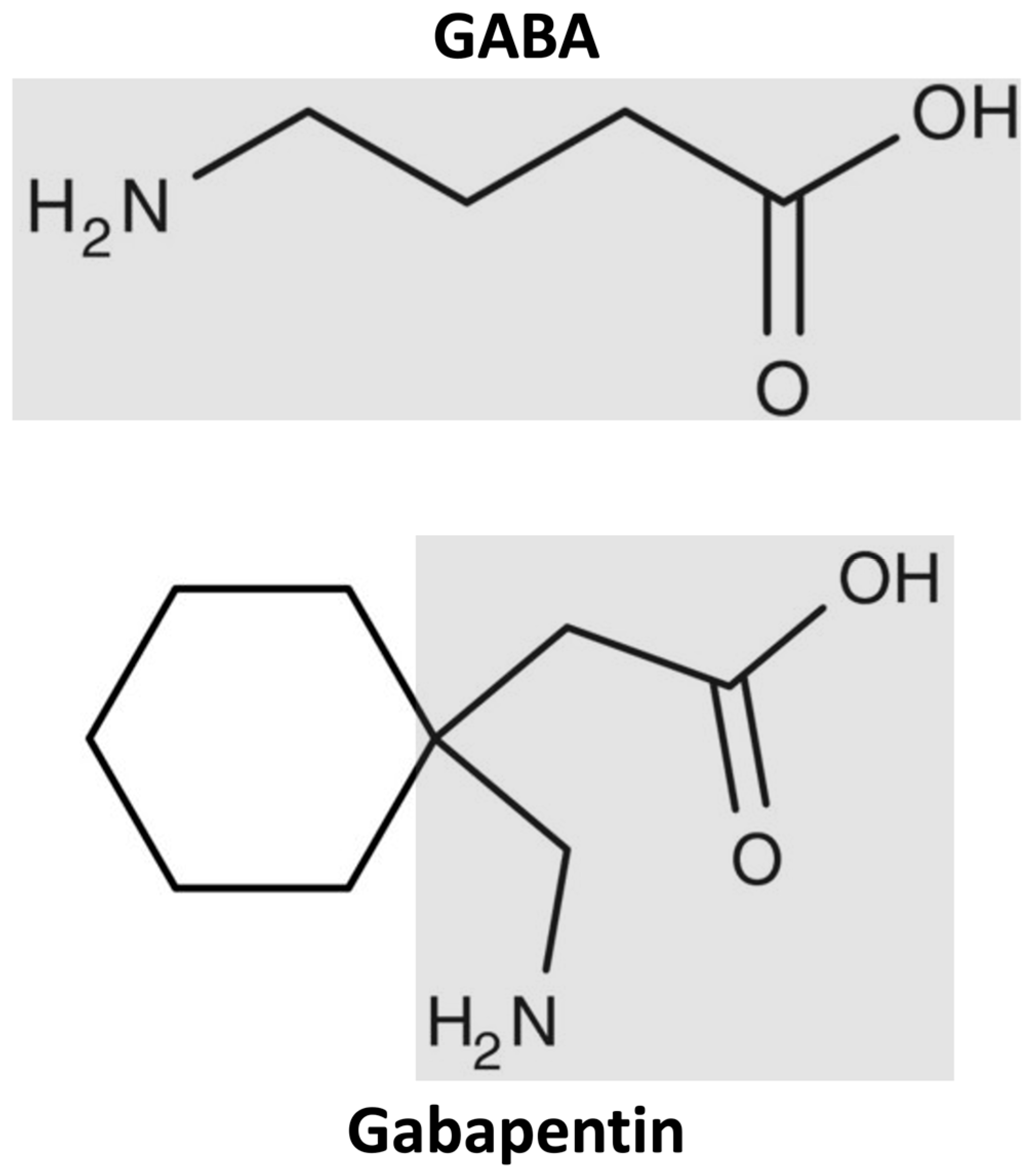
Abstract Context. Rising drug costs have increased focus on how new pharmaceuticals diffuse into the marketplace. The case of gabapentin use in bipolar disorder (BPD) provides an opportunity to study the roles of marketing, clinical evidence, and prior authorization (PA) policy on off-label medication use. A recent survey using the US-based TriNetX electronic health records network showed that gabapentin had been prescribed at least once in 13.6% of patients with bipolar disorder (BD), 11.5% DSM-IV criteria for unipolar major depressive disorder, bipolar disorder type I, and bipolar disorder type II. The diagnosis of bipolar disorder, NOS, was made using DSM-IV criteria augmented by the recommended criteria of Akiskal,13 in order to assess possible effects of gaba-pentin on a broad definition of the bipolar spectrum. Indi- Two new anticonvulsants, lamotrigine and gabapentin, have been used increasingly for bipolar disorder in the past several years. Despite this array of options, bipolar disorder remains a difficult disorder to treat. Some subtypes, such as those characterized by rapid cycling or mixed episodes, have been especially resistant to lithium treatment. Despite of the lack of evidence, reviews of gabapentin prescribing patterns in the United States show that this medication is still being used with alarming frequency for bipolar disorder. There are now five medications with specific, FDA approval for acute bipolar depression. Flowchart of included and excluded studies. Bipolar disorder (BD) Four DB-RCTs investigating the efficacy of gabapentin in BD were identified. 101 patients were randomised to receive gabapentin, 81 to placebo, 30 to lamotrigine and 19 to carbamazepine. is more gabapentin prescribed for bi-polar disorder than lamotrigine, even though there is little compelling evi-dence for gabapentin’s efficacy in bipolar disorder and the FDA has approved lamotrigine for the treat-ment of bipolar disorder.1,2 Thus, up to half of bipolar patients receiving combination therapy are given anti- Gabapentin is a medication that can be used to treat the symptoms of bipolar disorder, but like all medications, it can cause side effects. Some people may experience mild side effects when taking gabapentin, while others may experience more severe side effects. The drugs gabapentin and pregabalin are sometimes prescribed for people with bipolar disorder or insomnia. Research found little evidence that they are effective. The drugs have side effects and can be addictive; the team calls for further trials. Gabapentin and pregabalin (collectively known as gabapentinoids) are licensed in the UK to treat pain and seizures. RESULTS. Bipolar Disorder. The randomized controlled trials 19 –21 investigating gabapentin for treating bipolar disorder indicate it is likely to be ineffective. Data interpretation is difficult: dosing varies by trial, gabapentin is used as both monotherapy and adjunctive therapy, patients have heterogeneous diagnoses, and primary outcomes differ between studies. Gabapentin appears to have acute anti-manic and anti-depressant properties as an adjunctive agent for refractory bipolar illness. Prospective double-blind studies are needed to further delineate its acute efficacy when used as monotherapy and its prophylactic efficacy as monotherapy or in conjuction Researchers found that gabapentin does not help people with bipolar disorder. Learn more about the history of why some doctors prescribe gabapentin for bipolar as an adjunct therapy, even though there’s no evidence that it works for bipolar treatment or maintenance. We identified 40 open-label studies on the use of GBP in at least 600 patients with bipolar disorder (BP), manic, depressed, or mixed episodes and unipolar depression and four controlled studies. Gabapentin may be a useful drug for the add-on treatment of bipolar patients with poor response to other mood stabilizers. Gabapentin may improve depressive residual symptoms such as irritability, social withdrawal or anxiety. These results should be confirmed in randomized clinical trials. For bipolar disorder, four double-blind RCTs investigating gabapentin, and no double-blind RCTs investigating pregabalin, were identified. A quantitative synthesis could not be performed due to heterogeneity in the study population, design and outcome measures. Background: with increasing awareness of lithium's limitations, several new anticonvulsants had been tested for their mood stabilisation during recent years. Among the innovative third generation mood stabilizing anticonvulsants, gabapentin (GBP) seems to have a broad spectrum of efficacy, although no certain data are available as to its efficacy and use in clinical practice. Evidence does not support the use of gabapentin for bipolar disorder, major depressive disorder (MDD), posttraumatic stress disorder (PTSD), obsessive compulsive disorder (OCD), stimulant use disorder, or opioid withdrawal. Right now, there is no good evidence that gabapentin can be used for treating people with bipolar disorder. High-quality, randomized controlled studies found that gabapentin Case series suggest benefit of adjunctive gabapentin for mood symptoms in bipolar disorder, though the existing randomized controlled trials do not support this finding. Gabapentin’s role in acute mania is equivocal, and limited data exist on its use as prophylaxis in bipolar disorder. Abstract. Despite its prevalence and disease burden, several chasms still exist with regard to the pharmacotherapy of bipolar disorder (BD). Polypharmacy is commonly encountered as a significant proportion of patients remain symptomatic, and the management of the depressive phase of the illness is a particular challenge.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |