Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
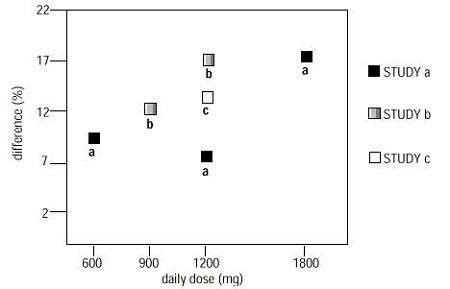
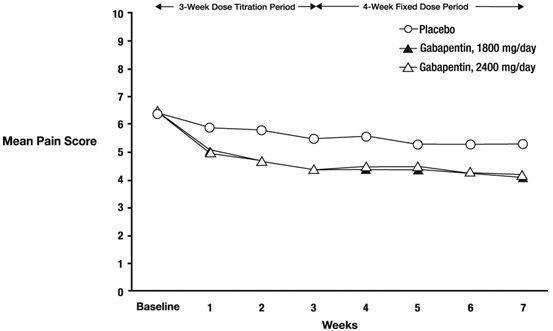
See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral Gabapentin oral solution is indicated for: Management of postherpetic neuralgia in adults - Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy ( 1) Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults (1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary The NDC Packaged Code 59762-5050-7 is assigned to a package of 1 bottle, plastic in 1 carton / 470 ml in 1 bottle, plastic of Gabapentin, a human prescription drug labeled by Greenstone Llc. The product's dosage form is suspension and is administered via oral form. Moreover, because gabapentin oral solution causes somnolence and dizziness [see Warnings and Precautions (5.4)], patients should be advised not to operate complex machinery until they have gained sufficient experience on gabapentin oral solution to assess whether gabapentin oral solution impairs their ability to perform such tasks. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults ( 1) (gabapentin) Oral Solution DESCRIPTION Neurontin ® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution The recommended maintenance dose of gabapentin oral solution in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin oral solution may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Patients taking Gabapentin Oral Solution should not drive until they have gained sufficient experience to assess whether gabapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended release) indicate that gabapentin may cause significant driving impairment. SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression (5.7) 4/2020 INDICATIONS AND USAGE Gabapentin oral solution is indicated for: (1) • • DOSAGE AND ADMINISTRATION • • • Page 8: Greenstone LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults Adjunctive therapy in the treatment of partial onset Stopping Gabapentin Oral Solution suddenly can cause serious problems. Gabapentin Oral Solution can cause serious side effects including: 1. Like other antiepileptic drugs, Gabapentin Oral Solution may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Use: Gabapentin oral suspension is used in the treatment of seizures, bipolar disorder, social phobia, and chronic pain. Packaging: Package in tight, light-resistant containers. Labeling: Shake well before taking. Neurontin® (gabapentin) oral solution DESCRIPTION Neurontin® (gabapentin) capsules, Neurontin® (gabapentin) tablets, and Neurontin® (gabapentin) oral solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral Neurontin ® Prescribing Information: Medication Guide: GABAPENTIN ORAL SOLUTION Neurontin ® Prescribing Information: Medication Guide: GABAPENTIN TABLETS, FOR ORAL USE: Neurontin ® Prescribing Information: Medication Guide: GEMFIBROZIL TABLETS, USP: Lopid ® Prescribing Information: Prescribing Information: GENTAMICIN SULFATE OPHTHALMIC The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. Administer gabapentin three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Gabapentin Oral Solution is a prescription medicine used to treat: z Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |