Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
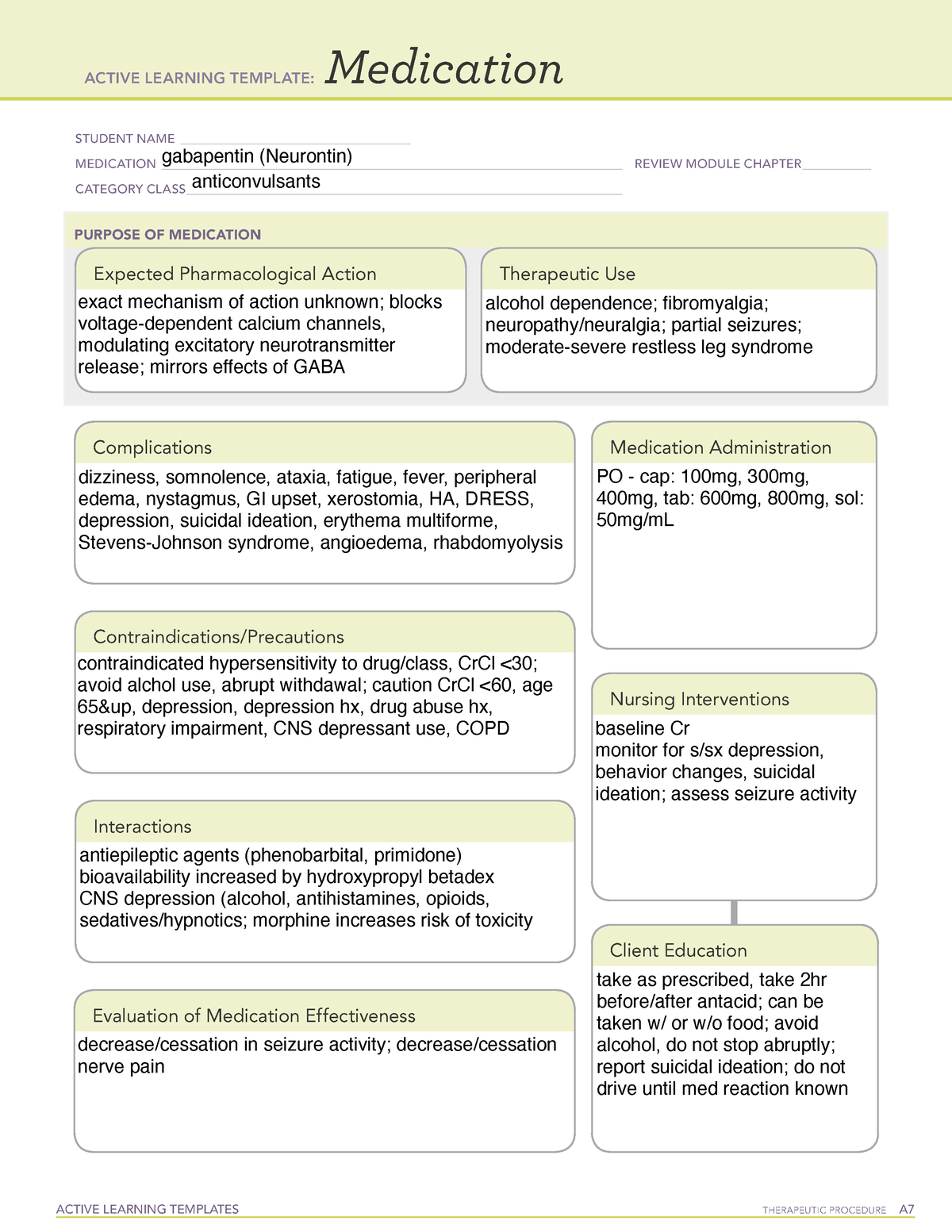 |  |
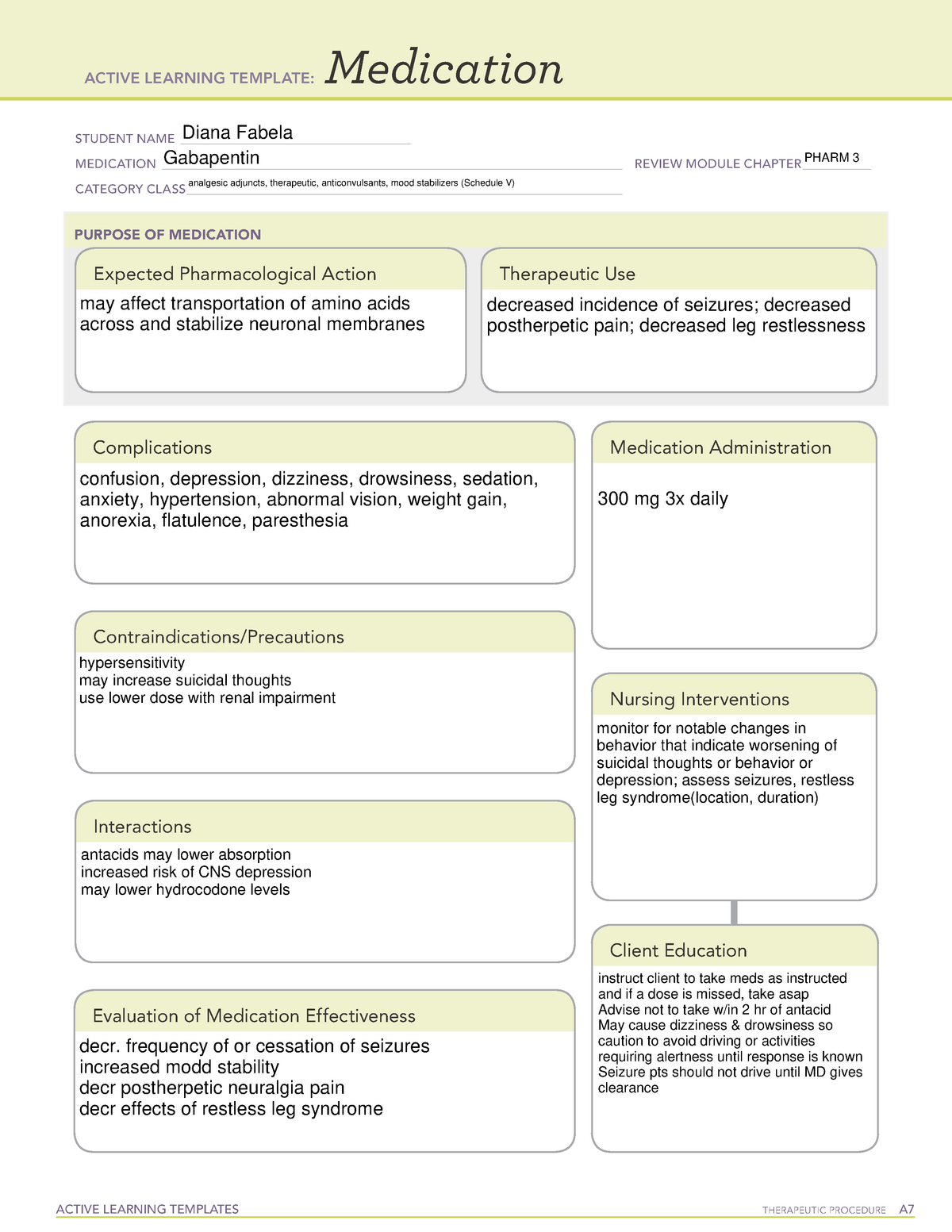
Schedule III, IV, or V — drugs with an abuse risk less than Schedule II. These drugs also have safe and accepted medical uses in the United States. Schedule III, IV, or V drugs include those containing smaller amounts of certain narcotic and non-narcotic drugs, anti-anxiety drugs, tranquilizers, sedatives, stimulants, and non-narcotic analgesics. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Gabapentinoids, also known as α2δ ligands, are a class of drugs that are chemically derivatives of the inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) (i.e., GABA analogues) which bind selectively to the α 2 δ protein that was first described as an auxiliary subunit of voltage-gated calcium channels (VGCCs). [1][2][3][4][5] Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. pursuant to the texas controlled substances act, health and safety code, chapter 481, these schedules supercede previous schedules and contain the most current version of the The drug has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence. The following drugs are listed as Schedule 2 (II) Drugs* by the Controlled Substances Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create Gabapentin belongs to a class of medications known as anticonvulsants or antiepileptic drugs (AEDs). Within this category, it stands out due to its unique mechanism of action compared to other traditional AEDs like phenytoin or carbamazepine. Find information on Gabapentin (Gralise, Horizant) in Davis’s Drug Guide including dosage, side effects, interactions, nursing implications, mechanism of action, half life, administration, and more. Gabapentin is available in 2 forms—gabapentin immediate release and the prodrug gabapentin enacarbil. Gabapentin is available in tablet form with strengths of 600 mg and 800 mg, capsules in strengths of 100 mg, 300 mg, and 400 mg, as well as an oral solution of 250 mg/5mL. Gabapentin belongs to a class of drugs called anticonvulsants. These drugs are used to treat a variety of conditions caused by abnormal electrical activity in the brain, including seizures, nerve pain, and restless leg syndrome. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] . Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin enacarbil (brand name Horizant) is a prodrug of gabapentin that has been designed to overcome the limitations of gabapentin, such as poor absorption and a short duration of action. It requires hydrolyzation in the gastrointestinal tract to become active. Gabapentin belongs to the group of medicines known as anticonvulsants. 2. Upsides
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |