Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
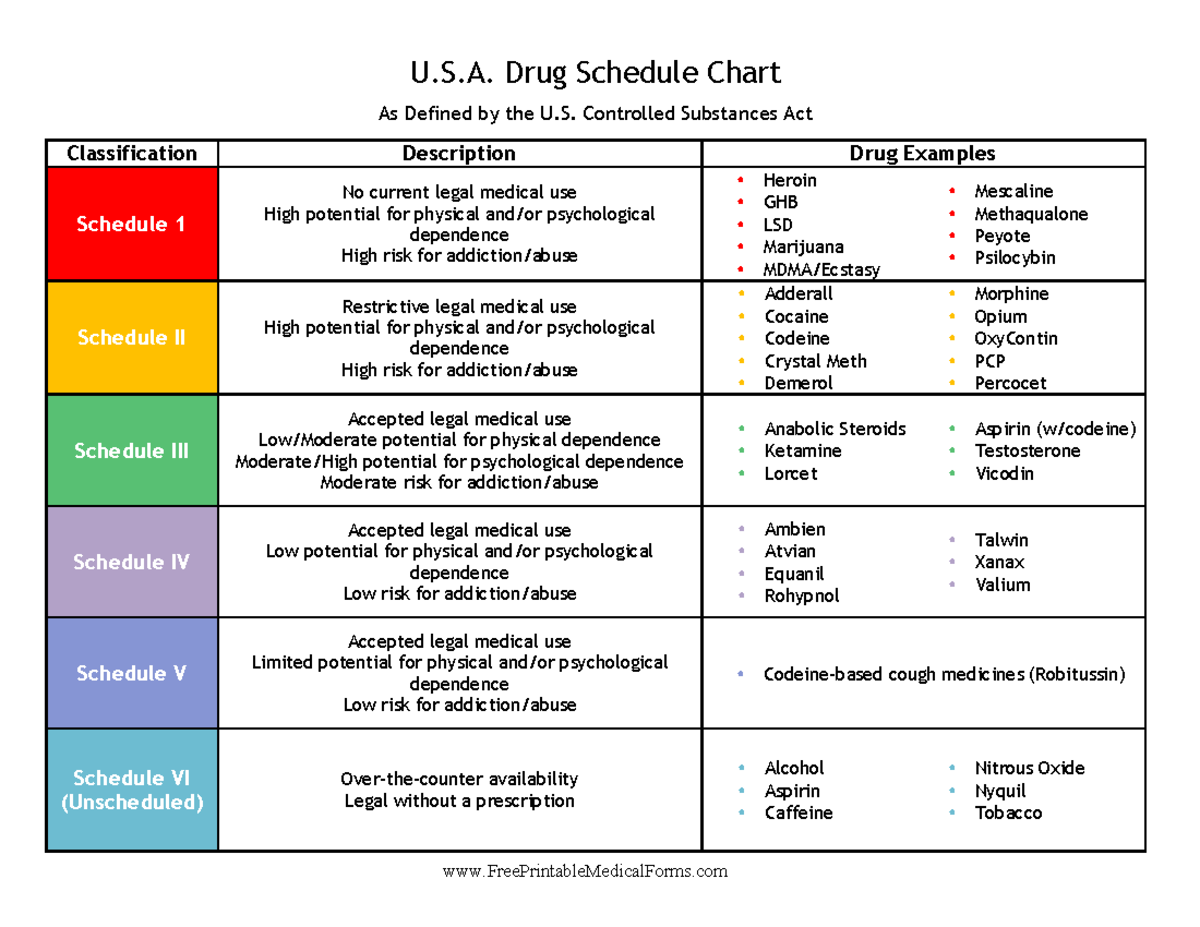
(a) [ (1)] A controlled substance may be dispensed only as provided in this section. Prescriptions electronically communicated must also meet the requirements under RCW 69.50.312. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Unless specifically excepted by state or federal law or regulation or more specifically included in another schedule, the following controlled substances are listed in Schedule IV: (a) [(1)] Any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or The controlled substances in this section may be added, rescheduled, or deleted as provided for in RCW 69.50.201. [ 2022 c 16 s 53 ; 2019 c 158 s 13 ; 2015 2nd sp.s. c 4 s 1203 ; 2010 c 177 s 2 ; 1993 c 187 s 4 ; 1986 c 124 s 3 ; 1980 c 138 s 1 ; 1971 ex.s. c 308 s 69.50.204 .] At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Gabapentin is classified as a controlled substance in several states due to its potential for misuse and abuse. Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. Currently, federal guidance is not available for misuse of gabapentin and the states hold the responsibility to oversee gabapentin, which range from no interventions to rescheduling the drug. The authors summarize that harm reduction needs to be through informed public policies to positively impact patient outcomes and enhance gabapentin safety. New opioid prescribing requirements are coming for many practitioners in Washington State. The goals are to increase public health and safety by reducing opioid abuse and misuse, while ensuring that practitioners continue to treat patients safely and appropriately for pain. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). RCW 70.225 Created Washington's Prescription Monitoring Program, required dispensers to report any Schedule II, III, IV, and V controlled substances and drugs of concern dispensed in Washington State or to an address in Washington to the WA PMP. The statute also authorized the Washington State Department of Health to establish rules for Unless specifically excepted by state or federal law or regulation or more specifically included in another schedule, the following controlled substances are listed in Schedule V: Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Retail pharmacies licensed with the Oregon Board of Pharmacy that dispense controlled substances in the state of Oregon, or to an address in the state, are required to electronically report prescription data. Neither hospital inpatient dispensing data nor data from veterinarians is collected. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. single substance pharmaceutical exposures (i.e., the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 6,955 cases in 2022. With respect to medical outcomes associated with gabapentin calls to poison control centers in 2022, gabapentin was associated with 6 deaths, 164 The State of Kentucky is, and to date, remains, the only state to have reclassified gabapentin as a Schedule-V controlled substance. 21 Effective July 1, 2017, the prescribing of gabapentin is limited to authorized practitioners, defined as practitioners registered with the US DEA. 21 Thus, mid-level practitioners, specifically, Physician (1) [(a)] Any compound, mixture, or preparation in dosage unit form containing any stimulant substance included in Schedule II and which was listed as an excepted compound on August 25, 1971, pursuant to the federal Controlled Substances Act, and any other drug of the quantitative composition shown in that list for those drugs or which is the same except for containing a lesser quantity of
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |