Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
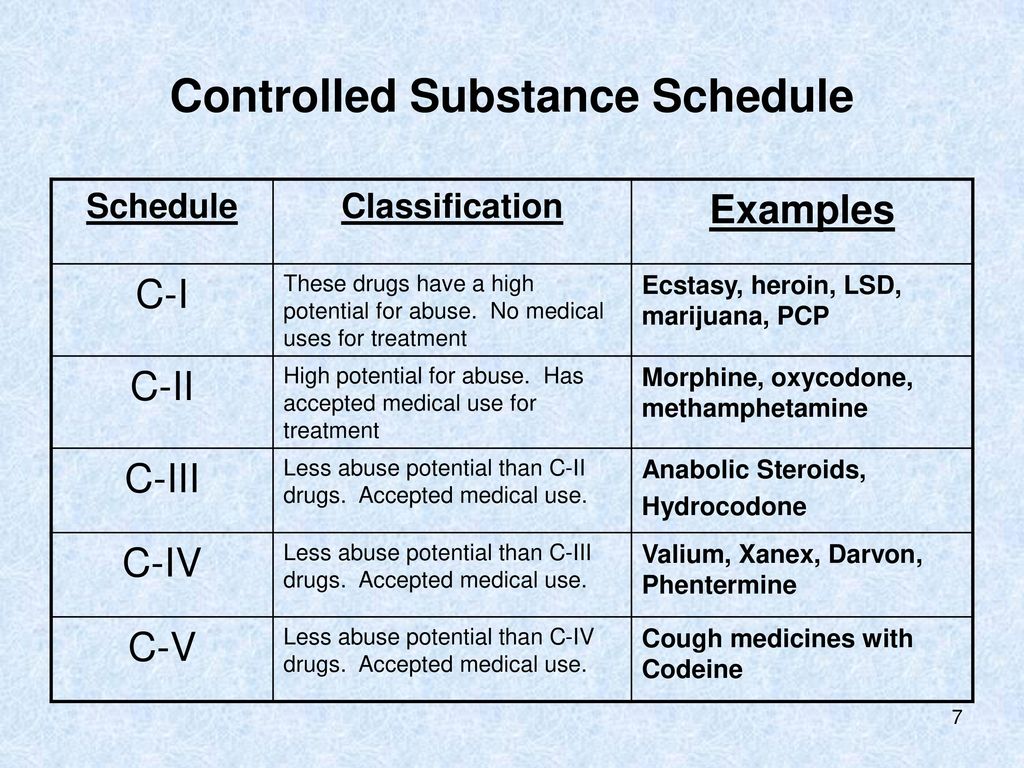
New Jersey doctors should not be caught flat footed when the Board starts cracking down on gabapentin. Physicians prescribing gabapentin must ensure their practices comply with all applicable laws and regulations on prescribing, despite the fact that gabapentin is currently an unscheduled drug. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. The NJPMP, established pursuant to N.J.S.A. 45:1-45 et. seq., is a statewide database that collects prescription data on Controlled Dangerous Substances (CDS), Human Growth Hormone (HGH) and gabapentin dispensed in outpatient settings in New Jersey, and by out-of-State pharmacies dispensing into New Jersey. The number of individuals who have Gabapentin present in an accidental overdose is significant enough . to add Gabapentin dispensation into the CPRMS. Note: The Department is not changing the controlled substance scheduling of Gabapentin at this time. As such, the CPMRS look-up requirements do not apply to Gabapentin prescribing. Naloxone pharmacy for a Schedule II, III, IV, or V controlled dangerous substance, for human growth hormone, or gabapentin dispensed to an inpatient at a hospital, long-term care, or other facility in which the resident is provided with 24-hour nursing care. In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance. 38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky To date, over 137 million controlled dangerous substance, human growth hormone and gabapentin prescriptions have been entered into the NJPMP from more than 3,300 New Jersey licensed pharmacies. The grant funds will further New Jersey’s efforts to prevent the abuse, misuse and diversion of controlled substances and will help prescribers make Established pursuant to N.J.S.A. 45:1-45 et. seq., the NJPMP is a statewide database that collects prescription data on Controlled Dangerous Substances (CDS), Human Growth Hormone (HGH), and gabapentin dispensed in outpatient settings in New Jersey, and by out-of-State pharmacies dispensing into New Jersey. Overseen by the NJ Division of Consumer Affairs Statewide electronic database for collecting data from outpatient pharmacies on: Controlled Dangerous Substances (CDS) Schedules II, III, IV and V Human Growth Hormone Products Gabapentin (May 7, 2018) Operational since September 1, 2011 In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Pursuant to N.J.S.A. 45:1-45 et. seq., and N.J.A.C. 13:45A-35.3, pharmacies that dispense Schedule II-V Controlled Dangerous Substances (CDS), Human Growth Hormone (HGH), and gabapentin in New Jersey, or into New Jersey, are required to submit data on all transactions for such drugs to the New Jersey Prescription Monitoring Program (NJPMP). Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is a crystalline substance and freely soluble in water, alkaline and acidic Gabapentin is not a controlled dangerous substance and currently is not required to be reported to the PMP. According to the Division, concerns have arisen in recent years over increasing instances of gabapentin abuse. A pharmacy filling prescriptions in New Jersey in an outpatient setting for a Schedule II, III, IV, or V controlled dangerous substance, for human growth hormone, or gabapentin. i. For purposes of this subchapter, "human growth hormone" means somatrem, somatropin, or any analogue of either of them, consistent with 21 U.S.C. § 333 (e)4; 2. Controlled Dangerous Substance . What is the criterion for a Schedule I controlled dangerous substance? The substance has high potential for abuse and has no accepted medical use in treatment in the United States, or it lacks accepted safety for use in treatment under medical supervision. (N.J.S.A. 24:21-5) Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |