Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 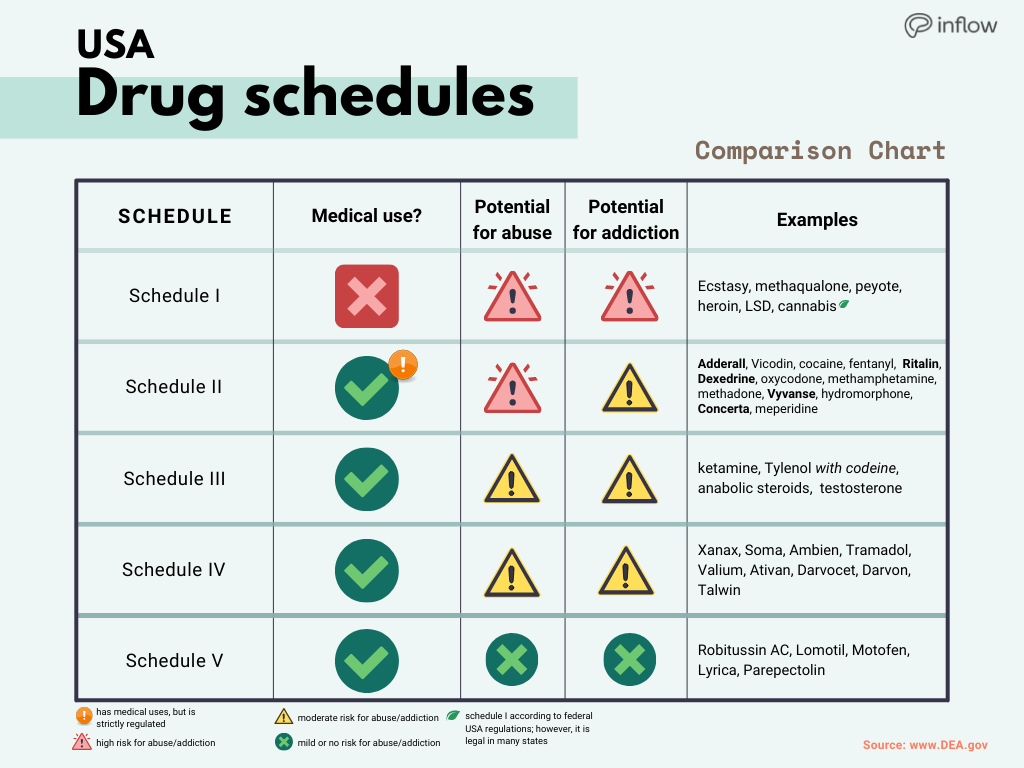 |
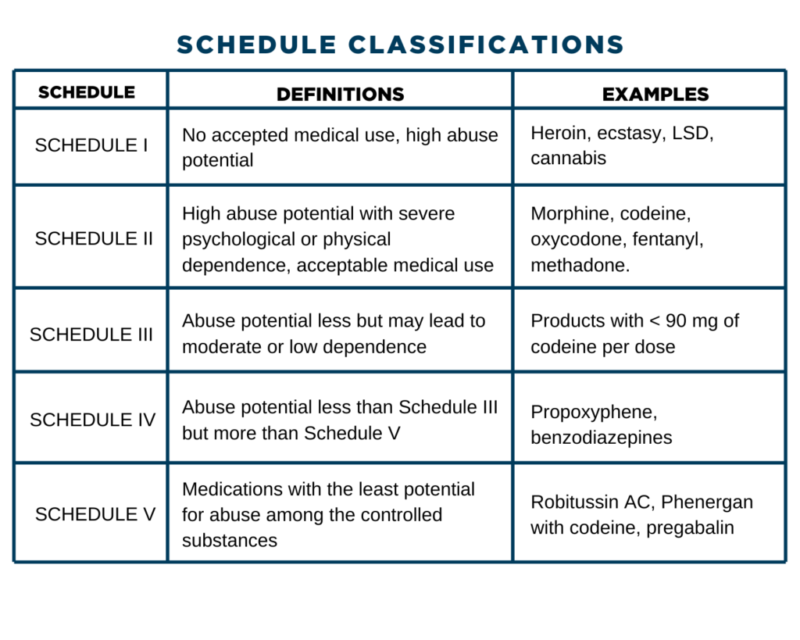
Name of targeted substances; Quantity and strength per unit of the targeted substance; Number of units per package; Name and address of supplier; Date received; Additional information. Any loss or theft of a narcotic, controlled drug, or targeted substance, including dispensed forgeries, must be reported to Health Canada within 10 days of Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. A monitored drug is a controlled substance as defined in the Controlled Drugs and Substances Act (Canada), unless the controlled substance has been excluded by the regulations under the Narcotics Safety and Awareness Act, 2010. Additional drugs may be specified as monitored drugs by the regulations. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Federal, provincial and territorial levels of government have roles to play in the treatment of substance use disorder and the delivery of a safer supply of controlled substances. In general, the relevant legislative and regulatory frameworks relate to controlled substances and health care service delivery. Controlled Substances (Drugs) Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Approved Drug Products containing Gabapentin listed with Health Canada. Original Data : Health Canada Website - Controlled Substance - Overview - Custom Synthesis Provincially, opioids and controlled substances (i.e., drugs in the schedules to the Controlled Drugs and Substances Act) are considered monitored drugs and subject to the Narcotics Safety and Awareness Act. Below is a list of resources to help pharmacists navigate and comply with the relevant legislation governing controlled substances. Learn how the Ontario health care system collects and stores information on prescribing and dispensing medications. The Narcotics Monitoring System (NMS) has been developed to collect and store information on prescribing and dispensing activities for these medications. , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. Gabapentin is frequently combined with other substances for the purpose of potentiating the effects of the drugs or achieving a “high.” Studies have identified various substances that are commonly abused in combination with gabapentin, including alcohol, opioids, benzodiazepines, antidepressants, and other CNS depressants 13,14,15. Role of the Office of Controlled Substances (OCS), Health Canada, regarding controlled drugs and substances, with links to information on licensing, legislation, physical security requirements, test kit registration, and loss or theft prescription drug pursuant to Wis. Stat. § 961.385 (1) (ag). Gabapentin is now listed in Wis. Admin. Code § CSB 4.03 (2) as a monitored prescription drug effective September 1, 2021. Gabapentin Has Not Been Scheduled as a Controlled Substance Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA In March 2013, the Canadian Centre on Substance Abuse (CCSA) released their report First Do No Harm: Responding to Canada’s Prescription Drug Crisis, which recognizes prescription drug abuse as the nation’s “leading public health concern.” Information about the product including what the product is used for, dosage, warnings, proper use and side effects. This summary will not tell you everything about the product. Contact your healthcare professional if you have any questions about the product. schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is a crystalline substance and freely soluble in water, alkaline and acidic • Few limits on the quantity, dose and frequency of narcotics and other controlled substances that can be dispensed in Ontario; • Lack of public awareness and education regarding the potential for abuse of these drugs; • No centralized database to record and monitor prescriptions for narcotics and other controlled substances in Ontario. Update on Gabapentin in Ohio As a reminder, gabapentin is not considered a controlled substance in Ohio. The Board was made aware of incorrect communications made by a third-party vendor stating that Ohio had made gabapentin a controlled substance. While gabapentin is not a controlled substance, rule 4729:8-2-02 requires the following entities to OTTAWA – Health Canada is advising Canadians about the increased risk of opioid overdose and serious side effects when taking gabapentin (e.g., Neurontin) or pregabalin (e.g., Lyrica) with an opioid. Gabapentin is authorized to treat epilepsy and pregabalin is authorized to treat nerve pain. In 2018, the U.K. reclassified the drug as a controlled substance along the same lines as opioids. Some states in the U.S. have mandated the reporting of gabapentin prescriptions.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |