Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 | |
 |  |
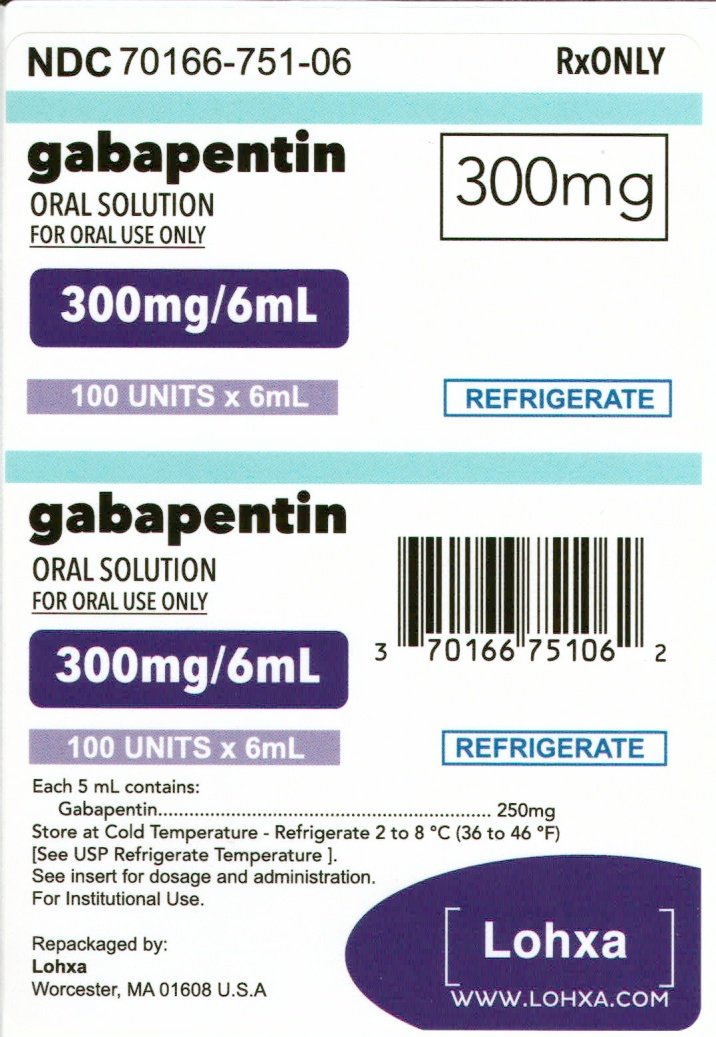
Effective July 1, 2017, all gabapentin products will be Schedule 5 controlled substances in Kentucky. All applicable provisions of KRS Chapter 218A, 902 KAR Chapter 55 and other licensure board regulations will apply to gabapentin. Justia Free Databases of U.S. Laws, Codes & Statutes. 2024 Tennessee Code Title 39 - CRIMINAL OFFENSES (§§ 39-1-101 — 39-17-1812) Chapter 17 - OFFENSES AGAINST PUBLIC HEALTH, SAFETY AND WELFARE (§§ 39-17-101 — 39-17-1812) Part 4 - DRUGS (§§ 39-17-401 — 39-17-456) Section 39-17-414 - Controlled substances in Schedule V Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. R 338.3125 - Gabapentin has been added to the schedule 5 drug list as a controlled substance. As a result of this change, any prescribers prescribing gabapentin must be registered with the Michigan Automated Prescription System (MAPS). Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. The drug has a currently accepted medical use in treatment in the United States. Abuse of the drug may lead to limited physical dependence or psychological dependence relative to the drugs in schedule 4. The following drugs are listed as Schedule 5 (V) Drugs* by the Controlled Substances Act (CSA): Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. But some states do control its use, labeling gabapentin as a Schedule 5 controlled substance. Why does gabapentin’s drug class vary from state to state? Although gabapentin isn’t The petition also pointed to studies suggesting that both gabapentin and pregabalin, which is marketed as Lyrica and is already classified as a Schedule 5 controlled substance, pose an addiction risk for patients with current or past substance abuse. The petition specifically cited a 2017 review in the journal European Neuropharmacology. § 90‑93. Schedule V controlled substances. (a) This schedule includes the controlled substances listed or to be listed by whatever official name, common or usual name, chemical name, or trade name designated. In determining that a substance comes within this schedule, the Commission shall find: a low potential for abuse • Prescriptions for gabapentin may include up to 5 refills and expire 6 months after the date issued. • Prescriptions for gabapentin may not be pre-signed or post-dated. How does moving gabapentin to Schedule 5 affect dispensing practitioners? • Only authorized practitioners may directly dispense controlled substances to patients. In January 9, 2019 – In an effort to continue to combat the opioid epidemic in Michigan, the Dept. of Licensing and Regulatory Affairs (LARA), with the support of the Michigan Board of Pharmacy, has modified its Pharmacy Rules to categorize Gabapentin as a Schedule 5 controlled substance. Schedule-V controlled substance and mandated reporting to PDMP. The State of Kentucky is, and to date, remains, the only state to have reclassified gabapentin as a Schedule-V controlled substance. 21 Effective July 1, 2017, the prescribing of gabapentin is limited to authorized practitioners, defined as practitioners registered with the US DEA. 21 Thus, mid-level practitioners, specifically pursuant to the texas controlled substances act, health and safety code, chapter 481, these schedules supercede previous schedules and contain the most current version of the The controlled substances listed in this Code section are included in Schedule V: (1) Any compound, mixture, or preparation containing limited quantities of any of the following narcotic drugs, or salts thereof, which also contains one or more nonnarcotic, active, medicinal ingredients in sufficient proportion to confer upon the compound, mixture, or preparation valuable medicinal qualities Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 | |
 |  |