Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
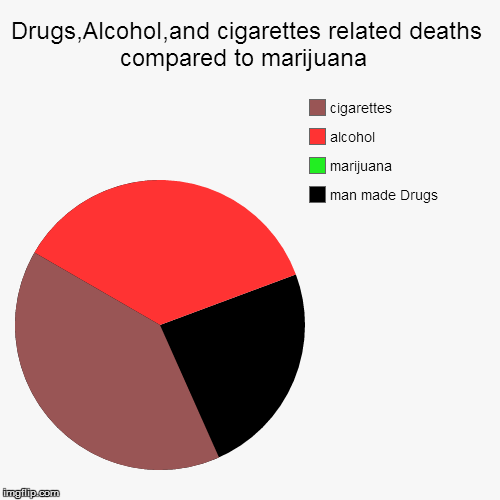
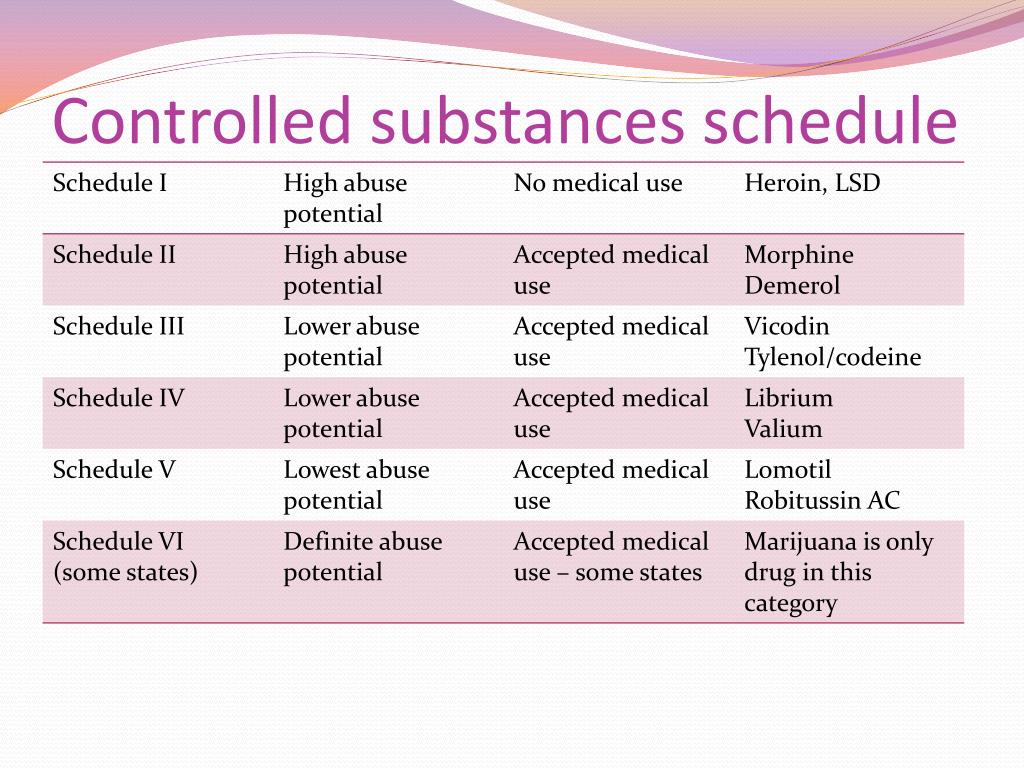
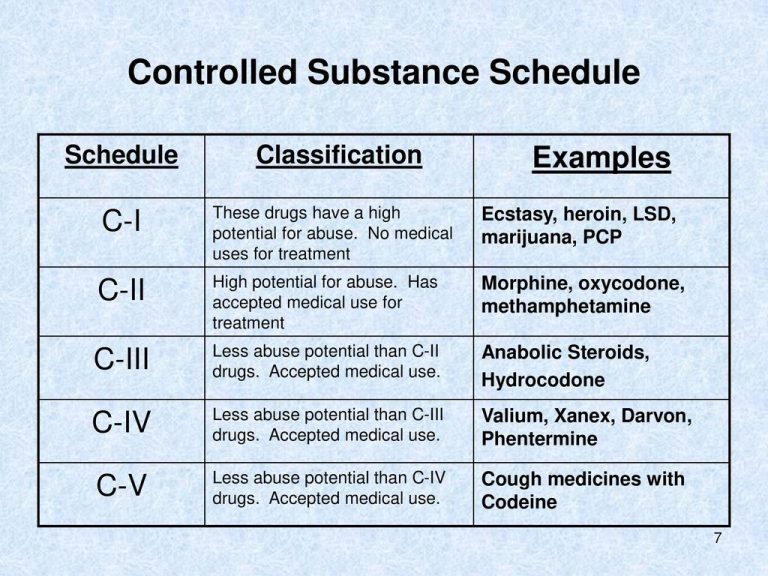
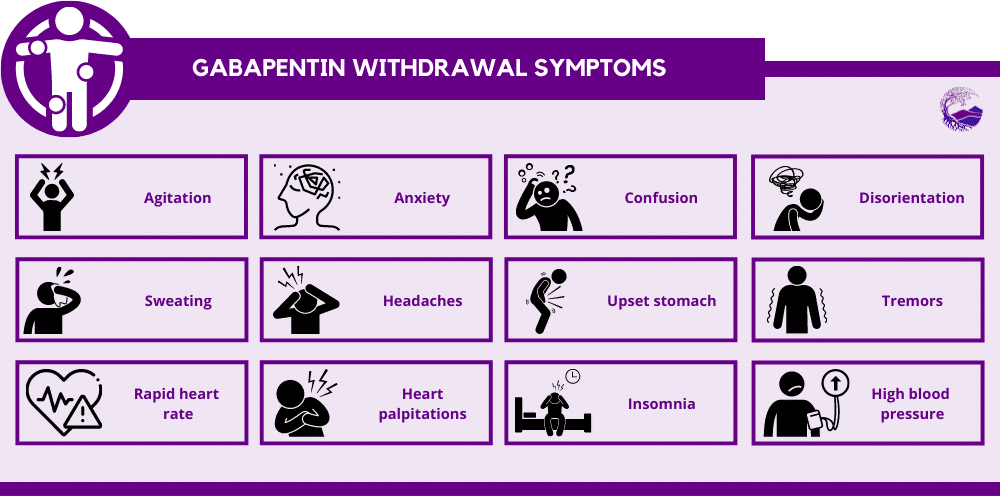
Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored 1 . Controlled Substances List (Adopted by Alabama State Board of Health . on February 22, 2024, effective February 22, 2024) Schedule I (a) Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, or brand name designated, listed in this section. Each drug or substance As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. Drugs DrugID SubstanceName DEANumbScheNarcoOtherNames 1 1-(1-Phenylcyclohexyl)pyrrolidine 7458 I N PCPy, PHP, rolicyclidine 2 1-(2-Phenylethyl)-4-phenyl-4-acetoxypiperidine 9663 I Y PEPAP, synthetic heroin 3 1-[1-(2-Thienyl)cyclohexyl]piperidine 7470 I N TCP, tenocyclidine 4 1-[1-(2-Thienyl)cyclohexyl]pyrrolidine 7473 I N TCPy purposes of this Schedule I hallucinogenic substances section only, the term “isomer” includes optical, position, and geometric isomers): (1) α-Ethyltryptamine (Other names: etryptamine; Monase; α- Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Taking gabapentin with other drugs that make you drowsy or slow your breathing can cause dangerous side effects or death. Ask your doctor before taking opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures. Tell your doctor about all your current medicines. Many drugs can affect gabapentin, especially: naproxen; Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Amendment adding drug products in finished dosage formulation that has been approved by the U.S. Food and Drug Administration that contains cannabidiol (2-[1R-3-methyl-6R-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol) derived from cannabis and no more than 0.1 percent (w/w) residual tetrahydrocannabinols to Schedule V Annual Review Completed for all Drug Entries on 9-15-2022 . Please be advised that the information contained in this table is compiled solely from reference works recognized and approved by the State Board of Pharmacy pursuant to rule 4729:9-2-01. Abbreviations Used in Reference Table C.S.A Schedules I Schedule I II Schedule II III Schedule III Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Gabapentin isn’t a controlled substance or narcotic on the federal level, but several states have passed laws to make it a Schedule V controlled substance. Gabapentin has risks and adverse effects, especially when combined with some other substances. Schedules I, II, III, IV, and V shall, unless and until added pursuant to R.S. 40:962, consist of the following drugs or other substances, by whatever official name, common or usual name, chemical name, or brand name designated: SCHEDULE I A. Opiates. Unless specifically excepted or unless listed in another schedule, any of the following Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |