Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
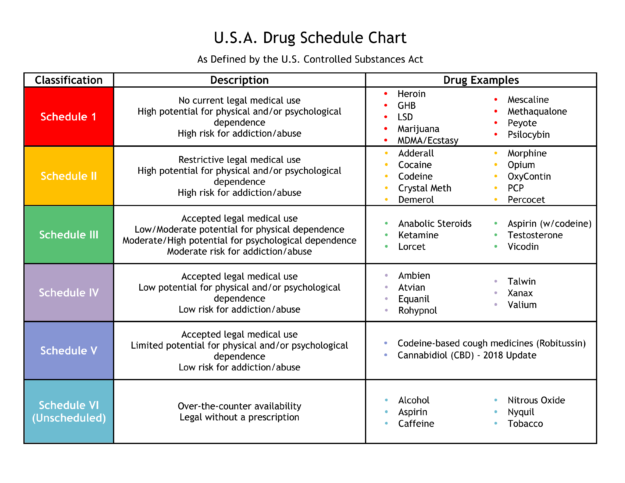
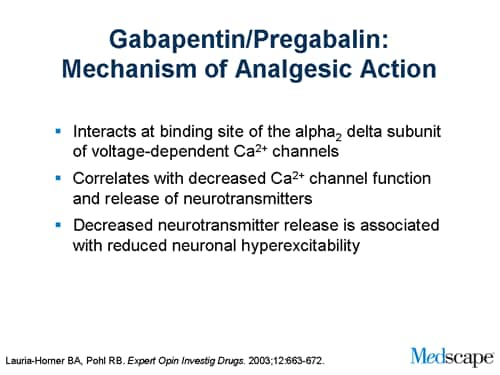
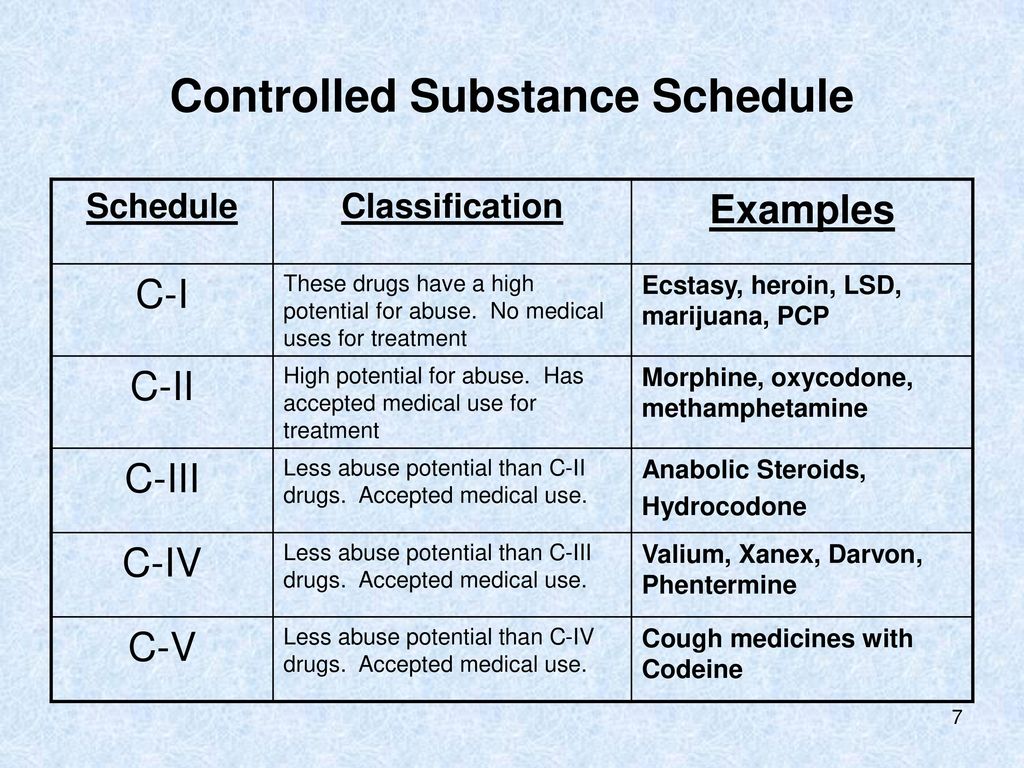
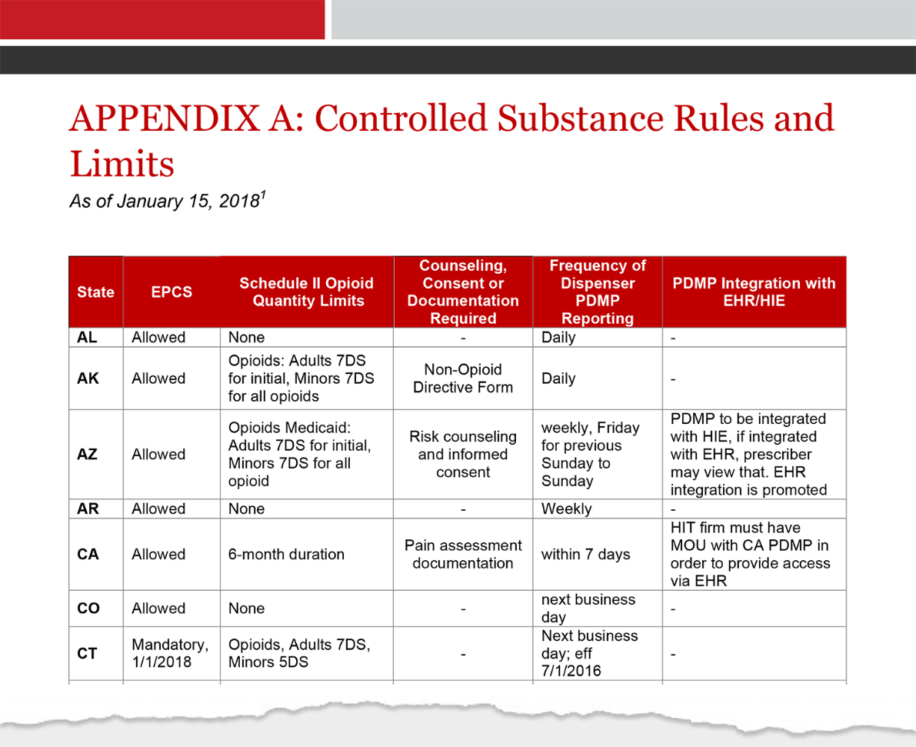
A written or electronic prescription for a controlled substance in Schedule II shall become invalid 30 days after the date of issuance. (b) A written or electronic prescription for a controlled substance in schedule II shall not be refilled. Written prescriptions for a controlled substance in schedule II shall be kept in a separate file. (c) Massachusetts enforces strict drug laws, categorizing controlled substances into different classes based on potential for abuse and medical use. These classifications determine criminal charges, penalties, and legal consequences for possession, distribution, and manufacturing. Please note, the Veterans Administration (VA) is limited by statute to report only dispensations of federally controlled substances in Schedules II-V. Accordingly, Gabapentin prescriptions filled at a VA facility will not appear on a patient’s PMP report. single substance pharmaceutical exposures (i.e., the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 6,955 cases in 2022. With respect to medical outcomes associated with gabapentin calls to poison control centers in 2022, gabapentin was associated with 6 deaths, 164 Pharmacies are required to submit dispensing information on federally controlled substances in Schedules II through V and gabapentin, a Massachusetts Schedule VI substance, within 24 hours or the next business day to the State of Massachusetts through the PMP Clearinghouse application provided by Bamboo Health, Inc. (Bamboo). Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). We register those who manufacture, distribute, dispense, possess, prescribe, or administer controlled substances. In Massachusetts, "controlled substances" includes all prescription drug products. Section 30 Administrative inspection of controlled premises; Section 31 Classes of controlled substances; establishment of criminal penalties for violations of this chapter; Section 32 Class A controlled substances; unlawful manufacture, distribution, dispensing or possession with intent to manufacture, etc.; eligibility for parole For the purposes of establishing criminal penalties for violation of a provision of this chapter, there are established the following five classes of controlled substances: CLASS A. Is Gabapentin a Controlled Substance in Massachusetts? Gabapentin, a medication commonly prescribed for nerve pain, is not currently classified as a controlled substance in Massachusetts. However, due to concerns about its potential for abuse, gabapentin is monitored by the state. In the case of gabapentin, it is the opposite. While gabapentin is NOT considered as a controlled substance on a federal level, there are some states that reclassified the drug as a controlled substance. The five states that have reclassified gabapentin as a controlled substance are Kentucky, Virginia, West Virginia, Michigan, and Tennessee. Gabapentin is frequently combined with other substances for the purpose of potentiating the effects of the drugs or achieving a “high.” Studies have identified various substances that are commonly abused in combination with gabapentin, including alcohol, opioids, benzodiazepines, antidepressants, and other CNS depressants 13,14,15. Last June, the Massachusetts PMP presented to the Mas-sachusetts Board of Registration in Pharmacy an update on efforts to integrate the Massachusetts Prescription Aware-ness Tool (MassPAT) directly into pharmacy software systems. Since then, the Massachusetts PMP has brought Walmart pharmacies, Boston Medical Center pharmacies, “The MCSR provides accountability for the manufacture, distribution, dispensing, possession, prescribing, and administering of controlled substances which, in Massachusetts, includes all prescription drugs.” To provide accountability for controlled substances, Massachusetts General Laws, Chapter 94C, section 7 and regulations of the Department of Public Health at 105 CMR 700.004 require every person who manufacturers, distributes, prescribes, administers, dispenses or possesses controlled substances to be registered with both the Department of Public Health and DEA if they utilize controlled Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Gabapentin and MassPAT The Massachusetts Prescription Awareness Tool (MassPAT) is an online tool utilized by authorized pro-viders that supports safe prescribing and dispensing of Schedule II-V controlled substances (CS). It is part of the Massachusetts Prescription Monitoring Program and requires pharmacies to report prescribing and dispensing
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |