Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
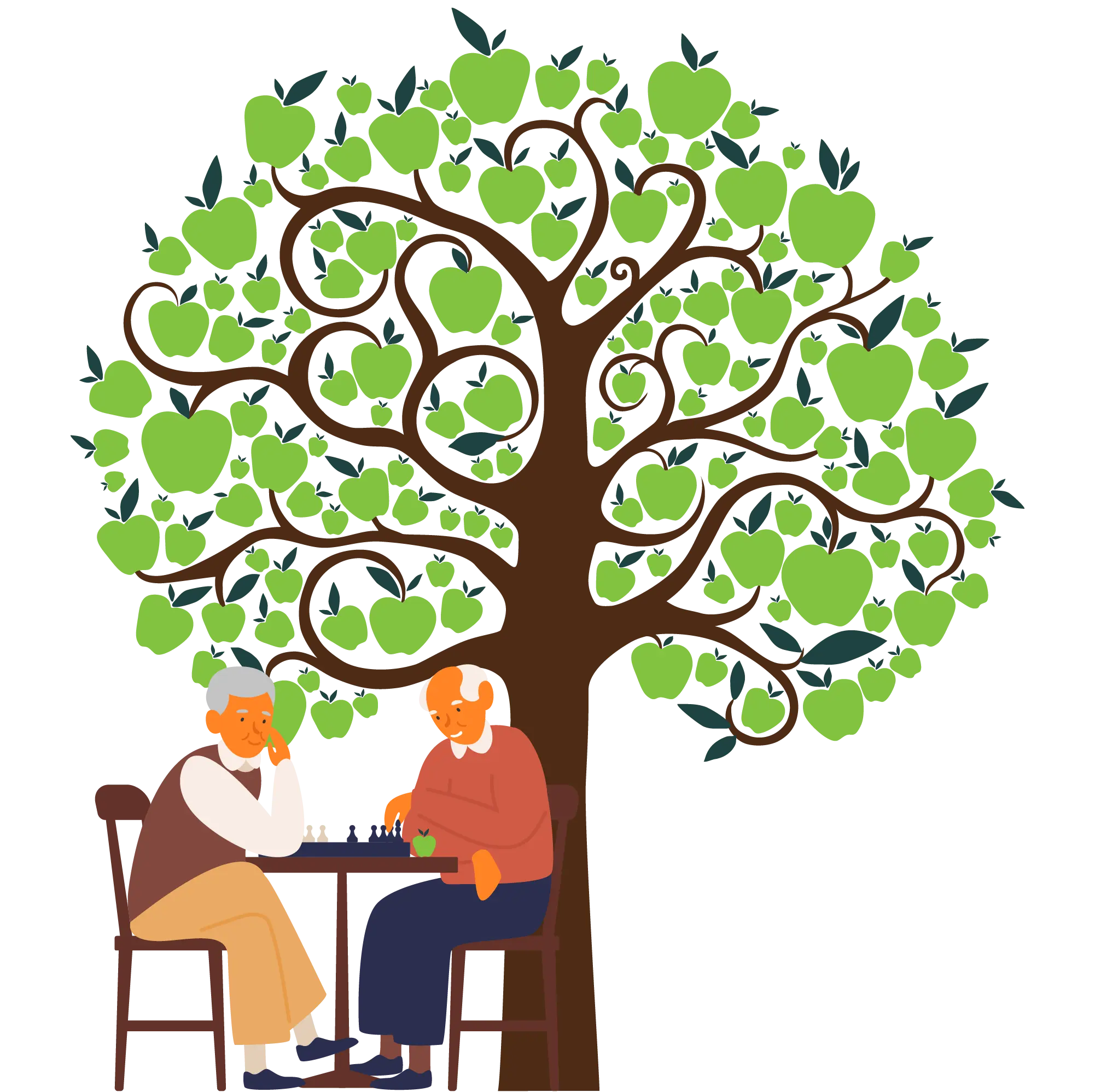
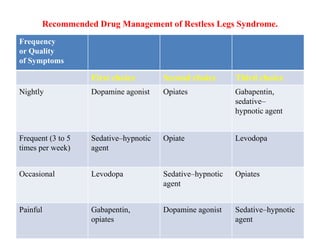
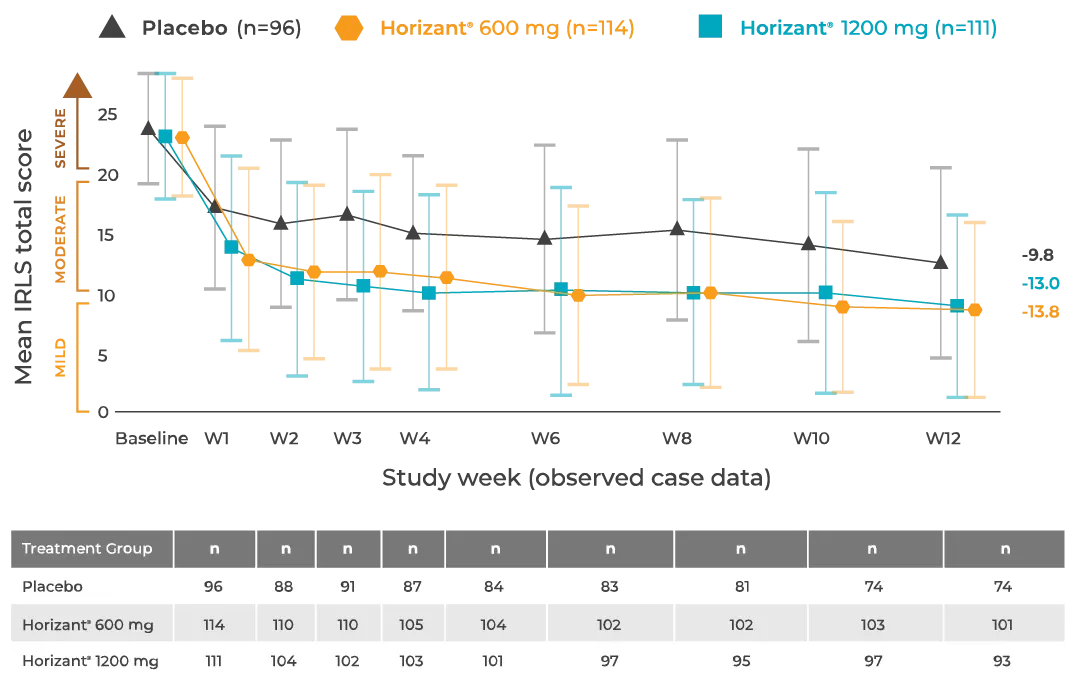
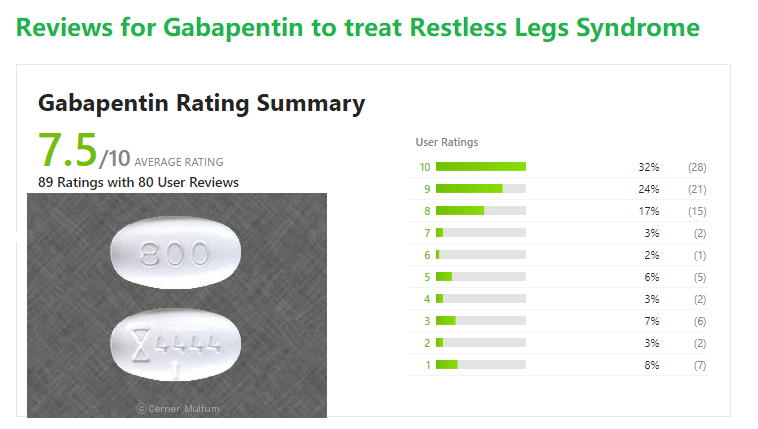
A. Gabapentin enacarbil (Horizant) has been approved by the FDA for the treatment of restless legs syndrome (RLS) and postherpetic neuralgia (the pain that can linger after a bout of shingles). It is different from plain gabapentin (Neurontin or Gralise). Restless legs syndrome (RLS) is a common disorder. The population prevalence is 1.5% to 2.7% in a subgroup of patients having more severe RLS with symptoms occurring 2 or more times a week and causing at least moderate distress. It is important for primary care physicians to be familiar with the disorder and its management. Much has changed in the management of RLS since our previous revised Other medications that may be effective include gabapentin, carbidopa/levodopa, opioids, and benzodiazepines. Restless legs syndrome may be a primary condition, or it may be secondary to iron In moderate to severe primary restless legs syndrome (RLS), clinicians should consider prescribing a pharmacologic agent to reduce RLS symptoms: Strong Evidence Pramipexole, rotigotine, cabergoline*, and gabapentin enacarbil ( Level A ). Gabapentin, primarily used for seizures and nerve pain, is also employed for Restless Legs Syndrome (RLS). It affects nerve signalling rather than muscles. Gabapentin’s effectiveness for RLS may take weeks, with dosage ranging from 300 mg to 3,600 mg daily. Objective: To assess the effects of gabapentin on sensory and motor symptoms in patients with restless legs syndrome (RLS). Methods: Patients with RLS (22 idiopathic, 2 secondary to iron deficiency) were randomized and treated for 6 weeks with either gabapentin or placebo. Gabapentin enacarbil available under the trade name Horizant is the only gabapentin product approved for treatment of Restless Legs Syndrome (RLS). A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose, but caused an increase in adverse reactions. This article explains what gabapentin is, its approved and off-label uses, and how the drug works to treat restless legs syndrome and other medical conditions. It also describes the possible side effects and risks and lists other drugs and treatments that may help ease RLS symptoms. Restless legs syndrome and cardiovascular disease: a research roadmap. Sleep Med. 2017;31:10–17. Crossref Google Scholar; 66. Migueis DP, Lopes MC, Casella E, Soares PV, Soster L, Spruyt K. Attention deficit hyperactivity disorder and restless leg syndrome across the lifespan: a systematic review and meta-analysis. Sleep Med Rev. 2023;69:101770. Gabapentin enacarbil is used to treat moderate-to-severe primary Restless Legs Syndrome (RLS). RLS is a neurologic disorder that makes the legs feel uncomfortable. This results in an irresistible feeling of wanting to move your legs to make them comfortable. Keywords: restless legs syndrome, pharmacotherapy, gabapentin, gabapentin enacarbil Introduction Restless Legs Syndrome (RLS) is a prevalent, disabling sleep-associated movement disorder requiring long-term drug therapy. To request a copy of the clinical practice guideline, “Treatment of restless legs syndrome and periodic limb movement disorder,” or the systematic review, meta-analysis, and GRADE assessment, or to arrange an interview with Dr. Winkelman or an AASM spokesperson, please contact the AASM at media@aasm.org. Accepted papers, which are published Restless legs syndrome (RLS) refers to an urge to move the legs, usually associated with unpleasant sensations. The urge to move the legs is worse at rest and at night and is relieved by movement. RLS is commonly associated with sleep disturbance and with involuntary, jerking movements of the legs during sleep, known as periodic limb movements Gabapentin has been shown to improve RLS in a small number of clinical studies, but is limited by its short half-life and variable bioavailability. Gabapentin enacarbil is a novel prodrug of gabapentin designed to overcome these pharmacokinetic limitations. Gabapentin enacarbil (marketed as Horizant) carries an FDA indication for the treatment of restless legs syndrome at a dose of 600 mg in the early evening, although FDA-approved doses of 1200 mg are permitted for other indications and used in some of the RLS clinical trials. The FDA approved gabapentin enacarbil in 2011 as the first non-dopaminergic agent for the treatment of restless legs syndrome (RLS) symptoms. Although gabapentin enacarbil is a pro-drug of gabapentin, its pharmacokinetics differ. Medicines such as gabapentin, gabapentin enacarbil and pregabalin are the first line of treatment for most people with RLS. These medicines can cause side effects such as dizziness, unsteadiness, mental fog and weight gain. Medicines that increase dopamine in the brain. These medicines affect levels of the chemical messenger dopamine in the brain. Restless legs syndrome (RLS) is characterized by an urge to move the legs, usually in association with limb discomfort. 1 The symptoms occur at rest, are relieved by movement, and are worse in the evening and at night.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |