Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
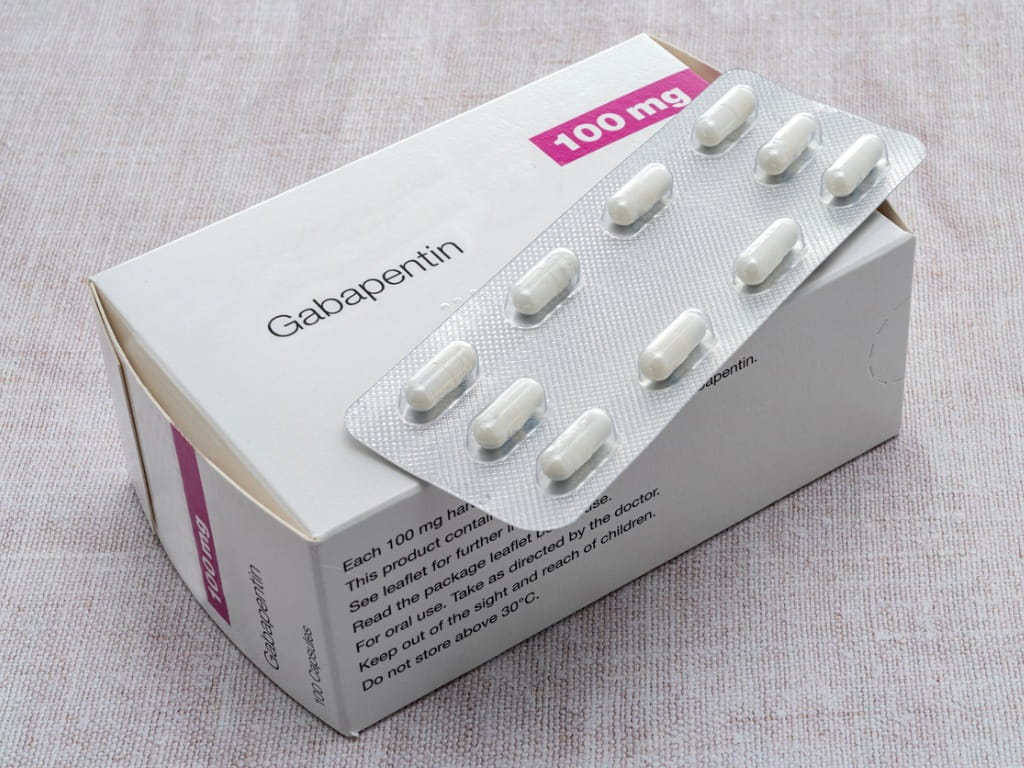
Gabapentin tablet is contraindicated in patients with demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS Once-daily gabapentin tablets are not interchangeable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The inactive ingredients for the capsules are corn starch, gelatin, magnesium stearate, manni Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. Neurontin (gabapentin) is used to treat pain you may have from shingles (postherpetic nerve pain). It is also used with other seizure medicines for partial onset seizures in patients 3 years and older. Gralise (gabapentin) is only used for pain after having shingles (postherpetic nerve pain). It should not be used for any other medical condition. Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). Known hypersensitivity to gabapentin or its ingredients (4) -----WARNINGS AND PRECAUTIONS----- Drug Reaction with Eosinophilia and Systemic Symptoms (Multiorgan hypersensitivity): Discontinue if alternative etiology is not established (5.1) Anaphylaxis and Angioedema: Discontinue and evaluate patient The active ingredient in NEURONTIN capsules, tablets, and oral solution is gabapentin, which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. The structural formula of gabapentin is: The inactive ingredients for the oral solution are glycerin, xylitol, purified water and artificial cool strawberry anise flavor. Gabapentin is described as 1-(aminomethyl)cyclohexaneacetic What are the ingredients in NEURONTIN? Active ingredient: gabapentin . Inactive ingredients in the capsules: lactose, cornstarch, and talc. The 100-mg capsule shell also contains: gelatin and titanium dioxide. The 300-mg capsule shell also contains: gelatin, titanium dioxide, and yellow iron oxide. What are the ingredients in NEURONTIN? Active ingredient: gabapentin . Inactive ingredients in the capsules: lactose, cornstarch, and talc. The 100-mg capsule shell also contains: gelatin and titanium dioxide. The 300-mg capsule shell also contains: gelatin, titanium dioxide, and yellow iron oxide. The inactive ingredients for the oral solution are glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. Gabapentin is described as 1-(aminomethyl) cyclohexaneacetic Store Neurontin capsules and tablets between 68°F to 77°F (20°C to 25°C). Store Neurontin oral solution in the refrigerator between 36°F to 46°F (2°C to 8°C). Keep Neurontin and all medicines out of the reach of children. What are the ingredients in Neurontin? Active ingredient: gabapentin. Inactive ingredients: Neurontin (gabapentin) is an anti-eleptic medication used to treat seizures that occur with epilepsy, as well as nerve pain associated with shingles. Learn side effects, dosage, drug interactions, warnings, patient labeling, reviews, and more. inal ingredients, or component of the container. For a complete listing, see 6 DO. function) and patients undergoing hemodialysis. (See 4.2 Recommended Dose and Dosage Adjustment, Special Patie. 00 mg tablets 3 times a day up to 1800 mg/ day. In clinical trials, the effective dosage range was 900 to 1800 mg/day, given 3 times a day using 300 mg. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly What are the ingredients in gabapentin? Active ingredient: Gabapentin USP . Inactive ingredients in the capsules: anhydrous lactose, cornstarch, and talc. The 100-mg capsule shell also contains: gelatin, sodium lauryl sulfate, and titanium dioxide. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. During the controlled epilepsy trials in patients older than 12 years of age receiving doses of gabapentin up to 1,800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). During the controlled epilepsy trials in patients older than 12 years of age receiving doses of gabapentin up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |