Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
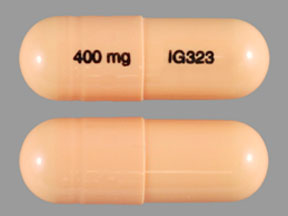
Gabapentin should only be used during pregnancy where benefits of treatment are considered to outweigh any potential risks. In view of the limited human pregnancy data, close monitoring of mother and fetus should be considered with use of gabapentin in pregnancy. The U.S. Food and Drug Administration classifies gabapentin (Neurontin) as a Pregnancy Category C medication, which means that animal studies conducted on this medication has caused harm on the fetus. If you're trying to get pregnant or have become pregnant while taking gabapentin, it is recommended to take a high dose of folic acid (5mg a day). You can get this from your doctor or midwife. Ideally you'll take high dose folic acid for 3 months before you start trying to get pregnant and for the first 12 weeks of pregnancy. Gabapentin is a γ-aminobutyric acid analog formally indicated for the treatment of epilepsy and neuropathic pain that is gaining increased popularity. Gabapentin has been historically considered a safe medication, including during pregnancy and lactation, with low reported concerns for misuse and use disorders. Gabapentin is a pregnancy category C, which means risk cannot be ruled out. Does gabapentin cause birth defects? There is currently not enough data to determine the risk of major birth defects in humans. 4,642 pregnancies with first trimester gabapentin exposure were identified (mean age of 28 years; 69% white). A reference group consisting of 1,744,447 unexposed pregnancies (mean age of 24 years; 40% white) was also identified. T1 exposure was defined as pregnancies with at least one filled prescription for gabapentin during the first 90 days of pregnancy (starting from the date of LMP), independently of exposure to gabapentin later in pregnancy. Medications approved prior to June 29, 2001 are not subject to the PLLR rule; however, the pregnancy letter category must be removed by June 29, 2018. For generic drugs, if the labeling of a reference listed drug is updated as a result of the final rule, the abbreviated new drug application (ANDA) labeling must also be revised. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early All pregnant women in the UK will be offered a very detailed anomaly scan at around 20 weeks of pregnancy as part of their routine antenatal care. No extra monitoring for major birth defects is required following gabapentin use in pregnancy. Babies exposed to gabapentin before delivery may experience withdrawal symptoms for a few days after birth. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Pregnancy categories A, B, C, D and X are being phased out. -Breastfed infants should be monitored for drowsiness, adequate weight gain, and developmental milestones, especially when used in combination with other anticonvulsant or psychotropic drugs and in younger, exclusively breastfed infants. The researchers reported 2 major malformations in infants exposed to gabapentin in the first trimester of pregnancy. 3 In another group of 7 women with hyperemesis gravidarum, 2 congenital defects were reported. 4 A cohort study in Denmark reported on 59 fetuses exposed to gabapentin during pregnancy, and documented 1 major malformation and 6 This sheet is about exposure to gabapentin in pregnancy and while breastfeeding. This information is based on available published literature. It should not take the place of medical care and advice from your healthcare provider. What is gabapentin? Gabapentin is a medication that has been used to prevent and control partial seizures, treat some forms [] There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as gabapentin, during pregnancy. Encourage women who are taking gabapentin during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling the toll free number 1-888-233-2334 or Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Small, controlled studies on gabapentin have not suggested an increased chance of birth defects. There is also no known pattern of birth defects associated with the use of gabapentin in pregnancy. Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. While gabapentin (Neurontin) is now used in a wide variety of clinical settings — for epilepsy, pain management, restless leg syndrome, anxiety, and sleep disturbance – there is relatively little information regarding its reproductive safety. Most recently, a prospective study from researchers at the Motherisk program reports on the outcomes of 223 pregnancies exposed to gabapentin Selected References: Blotiere PO, et al. 2020. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open 10(6). Brannon GE, Rolland PD. Anorgasmia in a patient with bipolar disorder type 1 treated with gabapentin. J Clin Psychopharmacol. 2000;20(3):379
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |