Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
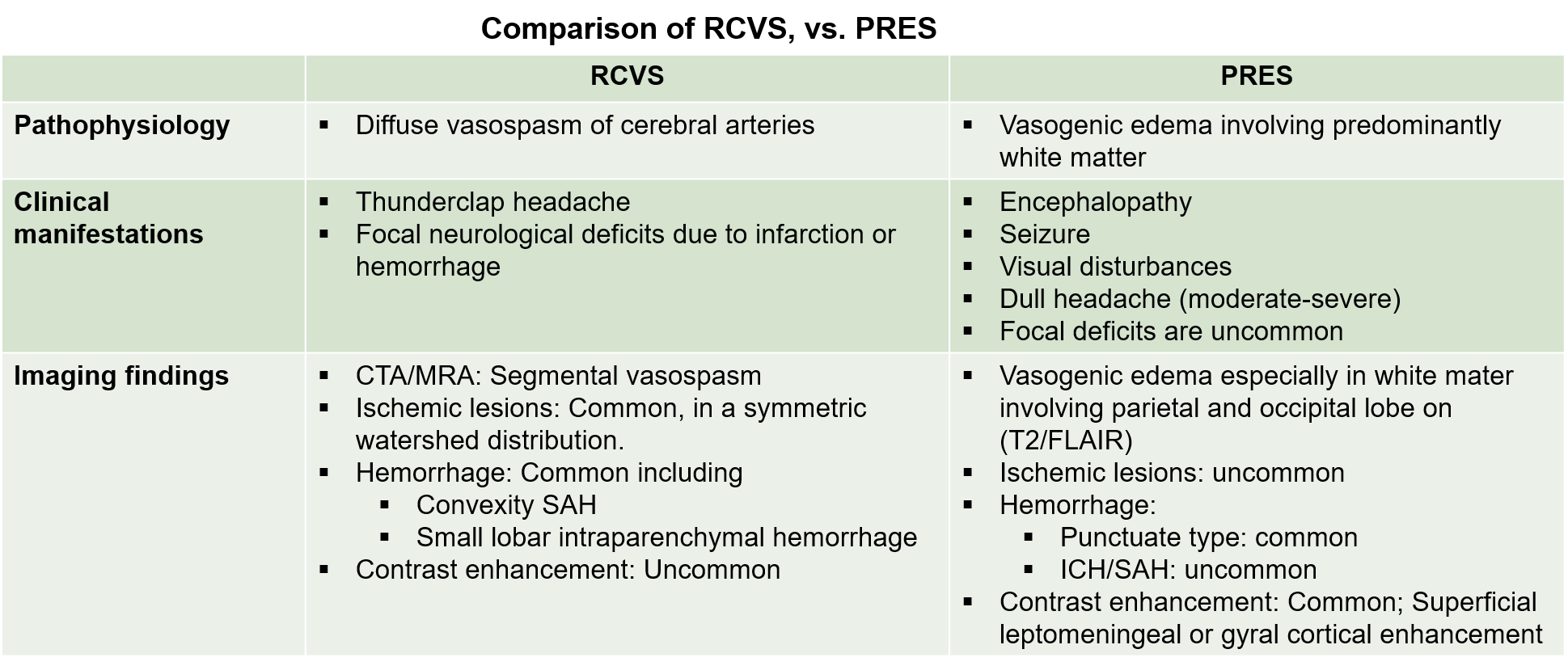 |  |
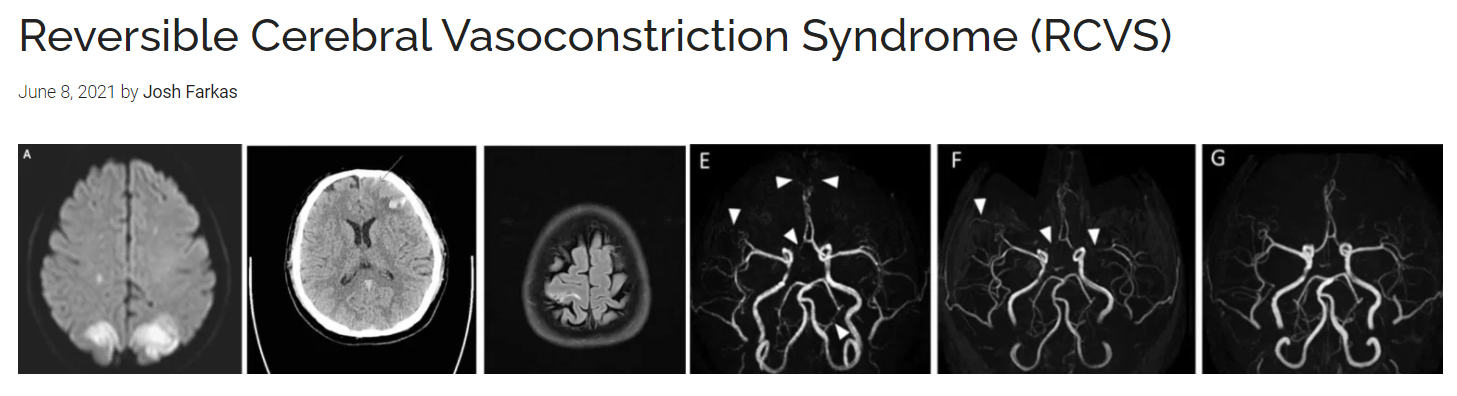
 | 70135-7/asset/52bc10c7-41a2-45ca-bd4a-de21c3932044/main.assets/gr1_lrg.jpg) |
As agreed on 7 September by RCVS Council, the implementation of the specific part of the new 'under care' guidance concerning the prescription of anti-parasitic POM-Vs came into effect on 12 January 2024 in order to help practices comply with the VMRs. The reclassification of pregabalin and gabapentin to Schedule 3 of the Misuse of Drug Regulations 2001 from 1 April 2019 will affect vets. These schedule 3 drugs: will be exempt from safe custody The Royal College of Veterinary Surgeons (RCVS) working with the Home Office and the VMD have produced general guidance to help vets comply with the Controlled Drugs legislation. Schedule 2 Examples of Schedule 3 CDs include tramadol, gabapentin, and pregabalin. Schedule 3 CDs are exempt from safe custody requirements, however, the RCVS advises that all Schedule 3 CDs should be stored in a CD cabinet. CDs in Schedule 4 are divided into two parts. Schedule 3: Includes tramadol, buprenorphine, pentazocine, gabapentin, pregabalin, the barbiturates and others. They are not legally subject to safe custody except buprenorphine, diethylpropion and temazepam which must be kept under safe custody (locked secure cabinet); but it is a Core requirement that all Schedule 3 drugs must be locked away. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. All CDs are listed in Schedules 1 to 5 of the Misuse of Drugs Regulations 2001. These are numbered in decreasing order of severity of control. The Schedules relate to the drugs’ therapeutic usefulness, the need for legitimate access and the potential harm caused by their misuse. The witness, if an independent veterinary surgeon, should record their RCVS number and confirm their independence in writing in the CD register. The VMD also say that the following information should be recorded: name of the CD, form, strength and quantity, and the signature of the professional destroying the drug. CDs are subject to additional legal requirements as they have been identified as at risk of abuse or misuse. For acute conditions, the maximum quantity of CDs prescribed should not exceed 30 days’ worth; exceptionally, to cover a justifiable clinical need and after consideration of any risk, a prescription can be issued for a longer period, but the reasons for the decision should be recorded Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Veterinary prescriptions for a schedule 2 or 3 controlled drug do not have to be on a standardised FP10PCD form, however certain legal requirements must be present for it to be legally valid. Below are the set of requirements specific to veterinary prescriptions and also requirements that are shared with controlled drug prescriptions for humans. Pregabalin and gabapentin will be reclassified to Schedule 3 from 1 April 2019, the Veterinary Medicines Directorate has confirmed. The move comes after experts highlighted increasing numbers of fatalities linked to the drugs, which are used to treat nerve pain, epilepsy and anxiety. In order to further assist vets in clinical practice with the changes to RCVS guidance on prescribing prescription-only veterinary medicines (POM-Vs) that were originally implemented on 1 September 2023 following the College’s 'under care' review, the RCVS and Veterinary Medicines Directorate (VMD) have together From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. As a schedule 3 controlled drug, there are additional controls on gabapentin that don’t apply to most other medicines. In most cases, an examination before prescribing is now a legal requirement. However, there are some limited exceptions permitted for ongoing treatment, as long as the patient has been stable on a set dose for long enough While all other Schedule 3 CDs, including tramadol, pentazocine, the barbiturates, gabapentin, and pregabalin, as well as Schedule 2 drug quinalbarbitone are not subject to the same Safe Custody Regulations, it is an RCVS requirement that they are securely locked away. From 1st April 2019, amendments to the Misuse of Drugs Regulations 2001 and the Safe Custody Regulations 1973 come into force which mean that pregabalin and gabapentin will be reclassified as Schedule 3 Controlled Drugs (CDs). 4.54 Although not all Schedule 3 controlled drugs are subject to the same legal safe custody requirements, it is an RCVS requirement that ALL Schedule 3 controlled drugs, for example tramadol, buprenorphine, pentazocine, the barbiturates, gabapentin and pregabalin (this list is not exhaustive), be securely locked away. 4.54 Although not all Schedule 3 controlled drugs are subject to the same legal safe custody requirements, it is an RCVS requirement that ALL Schedule 3 controlled drugs, for example tramadol, buprenorphine, pentazocine, the barbiturates, gabapentin and pregabalin (this list is not exhaustive), be securely locked away. Pregabalin and gabapentin reclassification Here we explain the prescribing and dispensing changes that will need to take place when pregabalin and gabapentin become Schedule 3 Controlled Drugs. Summary Requirements for gabapentin and pregabalin from 1st April 2019 are as follows: Controlled Drug Prescription requirements Prescription validity
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 70135-7/asset/52bc10c7-41a2-45ca-bd4a-de21c3932044/main.assets/gr1_lrg.jpg) |