Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
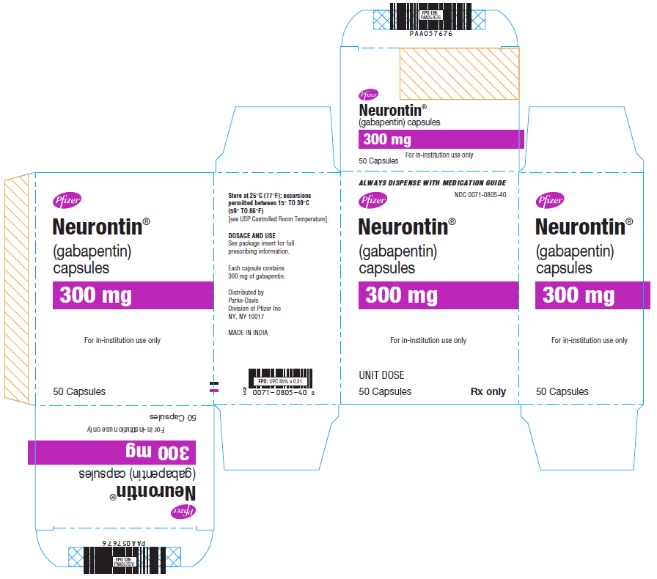 |
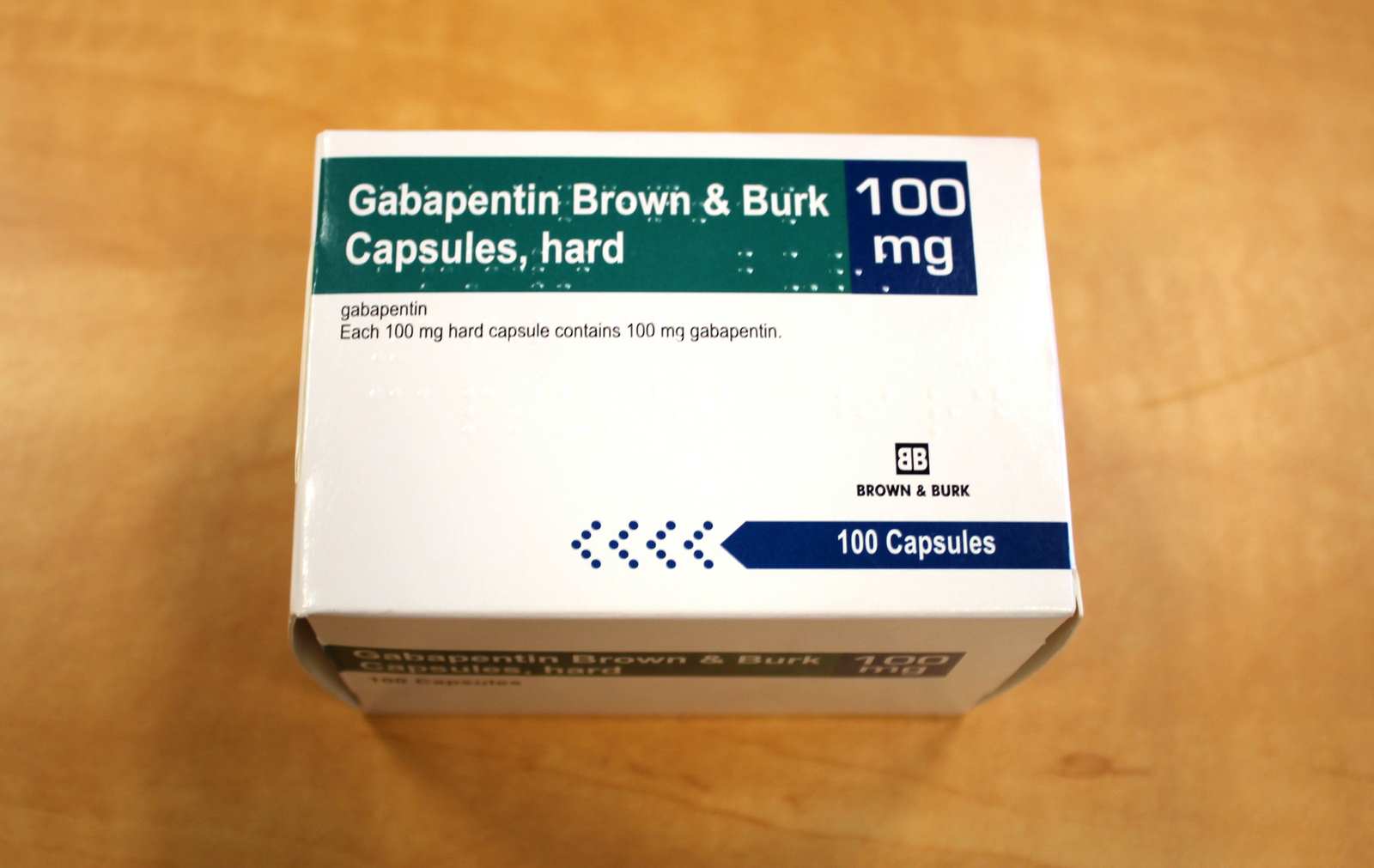
Known hypersensitivity to gabapentin or its ingredients (4) -----WARNINGS AND PRECAUTIONS----- Drug Reaction with Eosinophilia and Systemic Symptoms (Multiorgan ypersensitivity): Discontinue if alternative etiology is not established (5.1) •Anaphylaxis and Angioedema: Discontinue and evaluate patient Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Footnotes Footnote 1. The earliest marketed date recorded in the Drug Product Database. Return to footnote 1 referrer. Footnote 3. The American Hospital Formulary Service permits an easy review of information on a group of drugs with similar activities and uses and allows the reader to determine quickly the similarities and differences among drugs within a group. Tell your doctor immediately if you experience increased side effects such as drowsiness or slowed breathing while taking GABAPENTIN with an opioid or other sedatives and tranquilizers. The dose of these drugs or GABAPENTIN may need to be adjusted. Avoid alcoholic drinks while taking GABAPENTIN. The usual recommended adult dose of gabapentin begins with 300 mg 3 times daily. Your doctor may increase your dosage depending on how well it works and how well you tolerate it. The usual maximum daily dose is a total of 900 mg to 1,800 mg divided into 3 equal doses. Product monographs and consumer information for Teva Canada products can be found in the Health Canada Drug Product Database. Consult your healthcare practitioner if you have any questions about a medication you are taking. Product Monograph GABAPENTIN Page 1 of 31 PRODUCT MONOGRAPH PrGABAPENTIN Gabapentin Capsules (Manufacturer’s Standard) 100 mg, 300 mg and 400 mg Antiepileptic Agent Sivem Pharmaceuticals ULC 4705 Dobrin Street Saint-Laurent, Quebec H4R 2P7 www.sivem.ca Date of Revision: August 27, 2018 Submission Control No.: 219015 during TEVA-FEBUXOSTAT treatment, TEVA-FEBUXOSTAT should not be discontinued. The gout flare should be managed concurrently, as appropriate for the individual patient. TEVA-FEBUXOSTAT is not recommended for use in patients with a greatly increased rate of urate formation (e.g., malignant disease and its treatment, Lesch-Nyhan syndrome). Teva-Gabapentin: Gabapentin belongs to the class of medications called anti-epileptics. It is used in combination with other seizure control medications to manage and prevent seizures associated with epilepsy. Gabapentin does not cure epilepsy and only works to control seizures as long as the medication is taken. Gabapentin works by affecting the transmission of nerve signals in the brain. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Product name: TEVA-GABAPENTIN Company name: TEVA CANADA LIMITED DIN: 02244513 Status: Marketed Status date: 2010-04-21 Learn how the Teva Pharmaceutical Company pushes the boundaries of scientific innovation and delivers quality medication for millions of patients every day. PRODUCT MONOGRAPH PrTEVA-GABAPENTIN Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Teva Standard Teva Canada Limited 30 Novopharm Court, Toronto, Ontario Date of Revision: December 6, 2017 Canada M1B 2K9 www.tevacanada.com Submission Control No: 211039 TEVA-GABAPENTIN (gabapentin) Page 1 of 41 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrTEVA-GABAPENTIN Gabapentin Capsules Capsules, 100 mg, 300 mg, and 400 mg, Oral Gabapentin Tablets Tablets, 600 mg and 800 mg, Oral Teva Standard Product name: TEVA-GABAPENTIN Company name: TEVA CANADA LIMITED DIN: 02247346 Status: Marketed Status date: 2010-04-21 GABAPENTIN (gabapentin) Page 1 of 40 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrGABAPENTIN Gabapentin Capsules Capsules, 100 mg, 300 mg, and 400 mg, Oral Teva Standard Antiepileptic Agent Teva Canada Limited 30 Novopharm Court, Toronto, Ontario Product name: TEVA-GABAPENTIN Company name: TEVA CANADA LIMITED DIN: 02244514 Status: Marketed Status date: 2010-04-21 TEVA-GABAPENTIN (gabapentin) Product Monograph Page 5 of 31 Psychomotor Impairment Patients with uncontrolled epilepsy should not drive or handle potentially dangerous machinery. Patients taking TEVA-GABAPENTIN should not drive until they have gained sufficient experience Table 2. Dosage Adjustments for Renal Impairment in Adults Receiving Gabapentin Gastroretentive Tablets60; Cl cr (mL/minute). Adjusted Dosage Regimen. 30–60. 600 mg to 1.8 g once daily; initiate at 300 mg once daily and may titrate according to same schedule recommended for those with normal renal function based on individual patient response and tolerability GABAPENTIN- gabapentin capsule Actavis Pharma, Inc.-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN CAPSULES safely and effectively. See full prescribing information for GABAPENTIN CAPSULES. GABAPENTIN capsules, for oral use Initial U.S. Approval: 1993 INDICATIONS AND USAGE
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |