Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
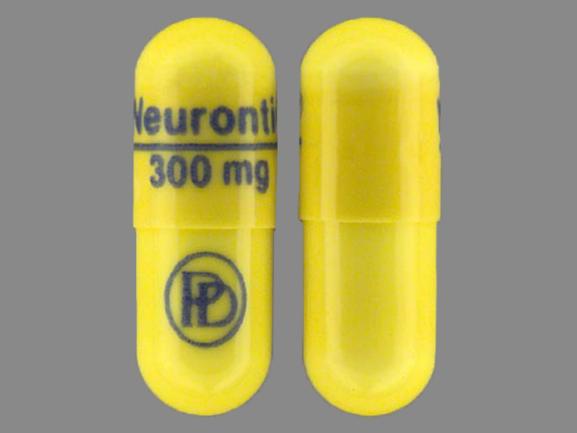
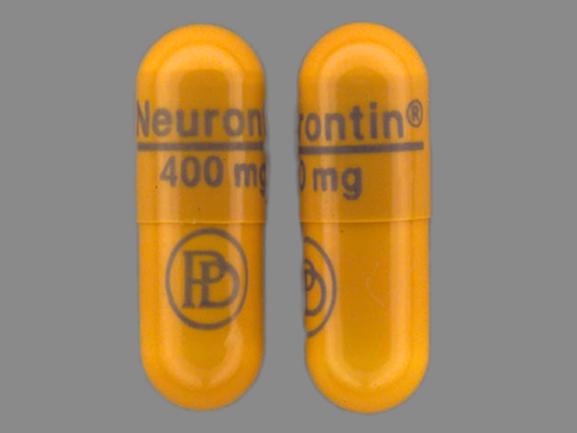
The inactive ingredients for the oral solution are glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. Gabapentin is described as 1-(aminomethyl) cyclohexaneacetic Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly The capsules also may contain lactose, cornstarch, and talc, and the oral solution may contain glycerin, xylitol, purified water, and strawberry-anise flavor. Gabapentin may be compounded into numerous dosage forms, including topical gels, transdermal gels, creams, and injections. Neurontin® Common Dosage Forms: Veterinary: None. Human: 100 mg, 300 mg, 400 mg, 600 mg, & 800 mg tablets or capsules. Compounded smaller sized capsules and a xylitol-free oral liquid may be available. This information sheet does not contain all available information for this medication and has not been reviewed by FDA Center for Veterinary Unfortunately, Neurontin® solution contains potentially toxic doses of xylitol, an artificial sweetener known to cause profound hypoglycemia and hepatic necrosis in canines. Gabapentin is also used quite frequently in felines at small doses, however it is unknown if cats have the same intolerance with xylitol as dogs. Neurontin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. All the best, The liquid form available in the USA usually contains glycerin, xylitol, purified water and artificial flavor. It is crucial to be aware that human formulations of liquid gabapentin may contain xylitol, which is highly toxic to cats. Therefore, it is absolutely essential to never give your cat human liquid gabapentin. If you’re sourcing Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal Gabapentin stabilizes electrical activity in the brain which prevents seizures caused by excessive electrical activity. Gabapentin mimics the activity of GABA (a neurotransmitter) which helps to calm the nerve activity in the brain. Storage: Store Gabapentin Oral Solution in the refrigerator between 36-46°F (2-8°C) NEURONTIN oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. *Note: Some human liquid oral formulations may contain xylitol, a sugar substitute. While this is toxic to dogs, it does not show the same response in rats at sub-acute levels. 1, 2, 3, 4. Pharmacology. Gabapentin is an analog of GABA (gamma-aminobutyric acid). As mentioned above, some liquid oral formulations of gabapentin contain xylitol, which is toxic to dogs. Be cautious and read the label before administering. Never give any medication to dogs that contain xylitol as an ingredient; Your vet may monitor your pet to be sure that the medication is working Although gabapentin does not contain xylitol, it is crucial to keep these two substances separate. Gabapentin is a medication commonly used to treat seizures and nerve pain, while xylitol is a sugar substitute often found in products like chewing gum, candy, and toothpaste. Patients taking gabapentin should not drive until they have gained sufficient experience to assess whether gabapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) gabapentin. Each gabapentin capsule, USP contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: corn starch, D&C red 33 (300 mg only), D&C yellow 10 (300 mg only), ferric oxide red (400 mg only), ferric oxide yellow (400 mg only), ferrosoferric oxide, gelatin, lactose monohydrate, potassium hydroxide, propylene glycol Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Other Names for this Medication: Neurontin® Common Dosage Forms: Veterinary: None. Human: 100 mg, 300 mg, 400 mg, 600 mg, & 800 mg tablets or capsules. Compounded smaller sized capsules and a xylitol-free oral liquid may be available. This information sheet does not contain all available information for this medication. In a table from the Manual for gabapentin’s use in dogs, there should be a warning of the risk of hepatic injury and/or failure and death with liquid solutions that contain xylitol, but there isn’t. Sedation, dizziness, ataxia, fatigue, diarrhea, reduce dose with renal dysfunction. Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: carboxymethylcellulose sodium, methylparaben, propylene glycol, propylparaben, purified water, xylitol, anise flavor, artificial strawberry flavor and hydrochloric acid added for adjustment of pH.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |