Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
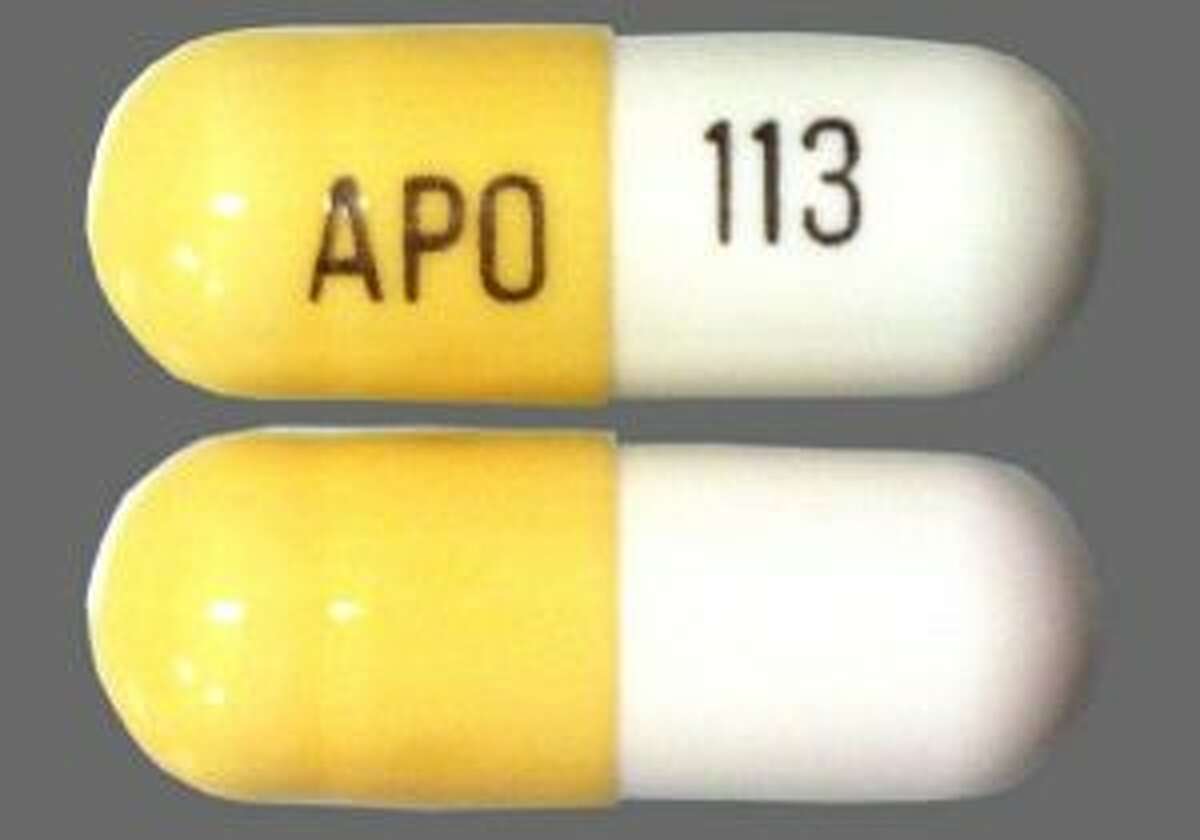
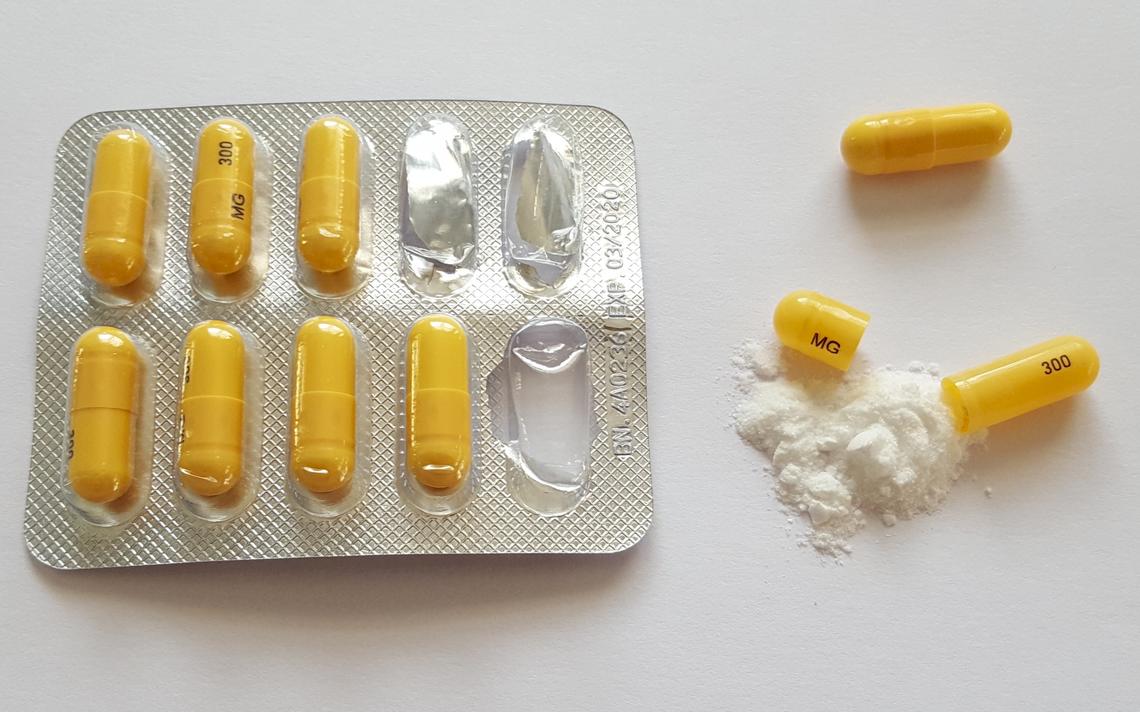
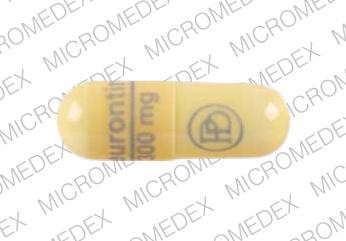
Classifies gabapentin as a controlled substance. law that includes non-narcotic drugs such as gabapentin. toring program. Section three establishes the effective date. Gabapentin is a popular prescription drug that is approved by the U.S. Food and Drug Administration (FDA) for nerve pain and epilepsy treat- ment. New York S8145 2017-2018 Establishes a schedule VI of drugs and other substances includes gabapentin as a drug to be monitored through the prescription monitoring program makes conforming changes and directs the commissioner of health to promulgate regulations necessary or desirable to classify gabapentin and its chemical equivalents as a scheduled substance for the purposes of article 33 of BILL NUMBER: S8145A SPONSOR: HANNON TITLE OF BILL: An act to amend the public health law, in relation to classifying gabapentin as a controlled substance PURPOSE: To establish a schedule VI of drugs and other substances, to include gabapentin as a drug to be monitored through the prescription monitoring program. Classifies gabapentin as a controlled substance. S T A T E O F N E W Y O R K _____ 10321 I N A S S E M B L Y April 10, 2018 _____ Introduced by M. of A. McDONALD -- read once and referred to the Commit- tee on Health AN ACT to amend the public health law, in relation to establishing a schedule VI of drugs and other substances, to including gabapentin as a drug to be monitored through the This bill reclassifies the drug gabapentin as a controlled substance in New York. It amends the public health law to add gabapentin to Schedule VI, which will require it to be monitored through the state's prescription monitoring program. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Gabapentin’s unscheduled status reflects its lower potential for abuse or dependency compared to controlled substances. However, the FDA monitors gabapentin for potential misuse, particularly when combined with other central nervous system depressants. This oversight aims to balance its therapeutic benefits against abuse risks. Regional Variation Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The number of states placing gabapentin on the controlled substance list or in their required monitoring program is growing and three more states are debating whether to add gabapentin as a controlled substance or to their mandated reporting programs (DE, NY, and WI). § 3306. Schedules of controlled substances. There are hereby established five schedules of controlled substances, to be known as schedules I, II, III, IV and V respectively. Such schedules shall consist of the following substances by whatever name or chemical designation known: Schedule I. (a) Schedule I shall consist of the drugs and other Effective August 18, 2016, Ioflupane, an injectable radiopharmaceutical diagnostic tool, that is derived from cocoa leaves, and is used in testing for adult patients with suspected Parkinsonism syndromes, was removed from Schedule II of the New York State Controlled Substance Schedules. New York S3906 2019-2020 Classifies gabapentin as a controlled substance Establishes a schedule VI of drugs and other substances; includes gabapentin as a drug to be monitored through the prescription monitoring program; makes conforming changes; and directs the commissioner of health to promulgate regulations necessary or desirable to classify gabapentin and its chemical equivalents as a scheduled substance for the single substance pharmaceutical exposures (i.e., the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 6,955 cases in 2022. With respect to medical outcomes associated with gabapentin calls to poison control centers in 2022, gabapentin was associated with 6 deaths, 164 11/01/2019: Gabapentin tracked by Utah Controlled Substance Database: HB 449 Rule R156-37f-203(8) Wyoming 25: 07/2017: Gabapentin reporting to WORx: W.S. 35-7-1001-1101 Future deliberations Delaware 26: Deliberations to classify gabapentin as a Schedule V controlled substance: HB 233: New York 27: Deliberations to classify gabapentin as a 80.71 Practitioners; dispensing controlled substances 71 80.72 Issuance of official New York State prescription forms 73 80.73 Pharmacists; dispensing schedule II substances and certain other controlled substances 74 80.74 . Pharmacists; dispensing schedule III, IV and V controlled substances 78 ARTICLE 33: NEW YORK STATE CONTROLLED SUBSTANCES ACT As of September 14th, 2020 Provided as a courtesy of Pharmacy Law Newsletter, University at Buffalo 5 5. "Controlled substance" means a substance or substances listed in section thirty-three hundred six of this chapter. 6. Patient reports will include all controlled substances that were dispensed in New York State and reported by the pharmacy/dispenser for the past year. This information will allow practitioners to better evaluate their patients' treatment with controlled substances and determine whether there may be abuse or non-medical use.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |