Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 | 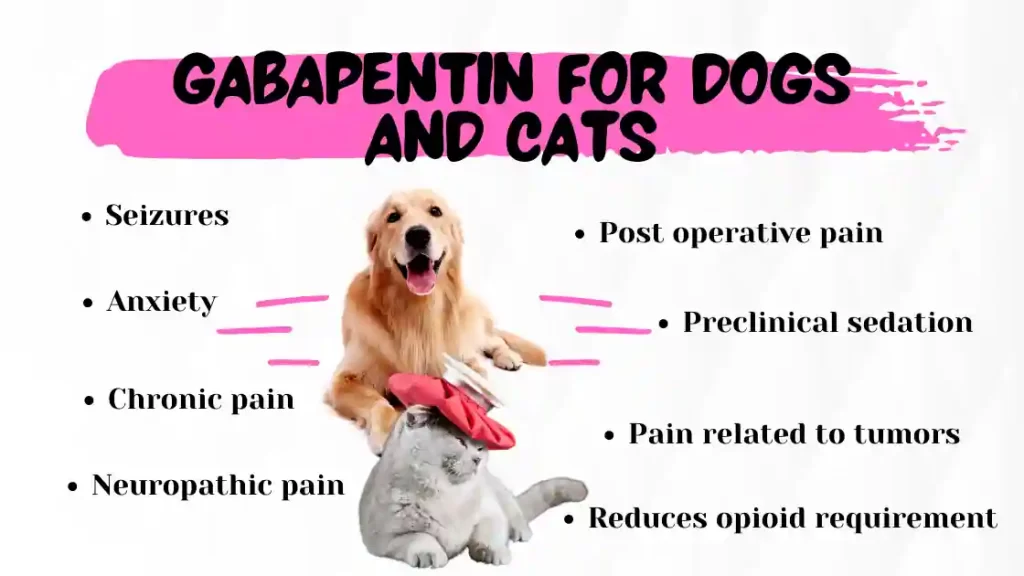 |
Some of these individuals were taking higher than recommended doses of gabapentin for unapproved uses. When prescribing gabapentin carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and drug-seeking behavior). Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly When used as directed, gabapentin is known to have numerous uses and benefits. It has been FDA-approved to help control and treat seizures and to diminish a specific type of nerve pain called Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. TABLE 1. Gabapentin Oral Solution Dosage Based on Renal Function. Pill with imprint IP 101 IP 101 is White, Capsule/Oblong and has been identified as Gabapentin 100 mg. It is supplied by Amneal Pharmaceuticals. Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Pill with imprint IP 102 IP 102 is Beige, Capsule/Oblong and has been identified as Gabapentin 300 mg. It is supplied by Amneal Pharmaceuticals. Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during Gabapentin Amneal may interfere with certain lab tests. Be sure your doctor and lab personnel know you are taking Gabapentin Amneal. Use Gabapentin Amneal with caution in the ELDERLY; they may be more sensitive to its effects, especially swelling of the hands, legs, or feet. Gabapentin Amneal works in the brain to prevent seizures and relieve pain for certain conditions in the nervous system. It is not used for routine pain caused by minor injuries or arthritis. Gabapentin Amneal is an anticonvulsant. Gabapentin Amneal is available only with your doctor's prescription. Gabapentin is a human prescription drug by Amneal Pharmaceuticals Llc. The product is distributed in 4 packages with NDC codes 65162-102-03, 65162-102-10, Gabapentin oral solution may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. Gabapentin capsules are indicated for: In adults with postherpetic neuralgia, gabapentin capsules may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). General information about the safe and effective use of gabapentin capsules. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use gabapentin capsules for a condition for which it was not prescribed. Do not give gabapentin capsules to other people, even if they have the same symptoms that you The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. These individuals were taking higher than recommended doses of Gabapentin Amneal for unapproved uses. Most of the individuals described in these reports had a history of poly-substance abuse or used Gabapentin Amneal to relieve symptoms of withdrawal from other substances. We are also excited about our work to bring patients more affordable biologic therapy options through our Biosciences, which includes a broad portfolio of institutional injectables as well as Amneal’s growing biosimilars portfolio. And we’re expanding our business across select international markets. Learn more about our product portfolios: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |